Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
Nick and Joris break down what a DHF is, why it's required, and how it plays a vital role throughout the development lifecycle.
-
Nick Allan and Joris van der Heijden revisit one of StarFish Medical’s most successful Pathfinder journeys, showcasing how a bold research concept evolved into a fully realized clinical diagnostic device.
-
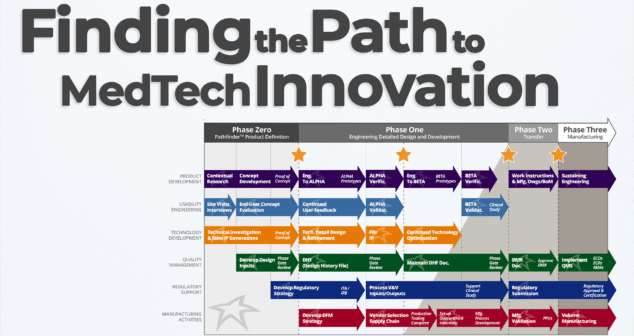
Nick and Joris explore one of the most dynamic early-phase services at StarFish Medical: the Pathfinder Program. If you're a medtech innovator with a promising concept or prototype, Pathfinder helps you identify the right path forward—before you invest millions in development.
-
Optics Physicist and Engineer share approaches to performing pre-screen Ophthalmic Instrument Safety Assessment testing in-house.
-

If you’ve ever been to the hospital, you’ll know that one of the first things hospital staff do is attach “that finger clip device” to your finger. “That device” is called a Pulse Oximeter, and it provides information on pulse rate and blood oxygenation.
-
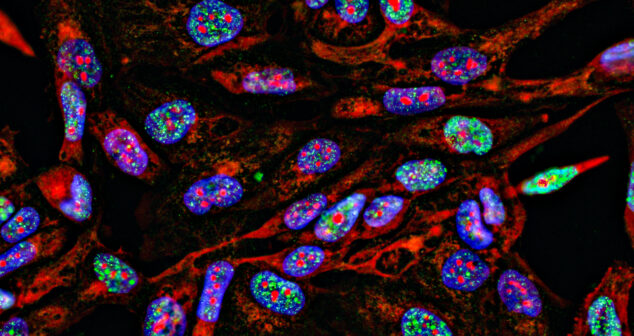
Fluorescence Imaging in Medical Devices outlines medical applications and examples of devices that use fluorescence for imaging.
-
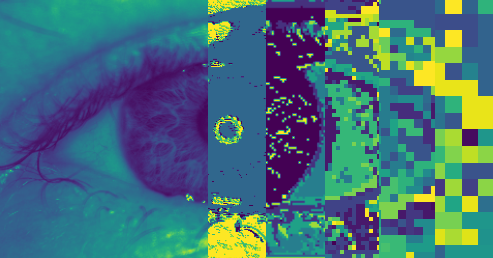
Computer Vision for Medical Devices is constantly evolving and incorporating new techniques and technologies as they emerge.
-
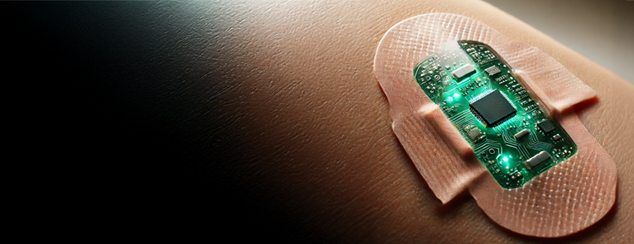
With the recent developments and seemingly ubiquitous nature of real time glucose monitoring and availability of smart wearable tech, the development of a theranostic band-aid seems inevitable. But how practical would this be? Is there a Theranostic wound dressings market?
-

In this episode of MedDevice by Design, Ariana Wilson and Mark Drlik examine what happened, what it means for medical device innovators, and how the FDA’s ASCA (Accreditation Scheme for Conformity Assessment) program helps reduce regulatory risk.