
Bio Break: The Science of Freeze-Drying
Nick and Joris dive into the fascinating world of freeze-drying, exploring how this process extends shelf life and maintains the integrity of various products—including reagents used in in vitro diagnostics and even instant coffee!
Nick starts off conducting a personal experiment with freeze-dried coffee, comparing its taste and texture to fresh-brewed coffee. But what does coffee have to do with medical device development? The connection lies in the freeze-drying (lyophilization) process, a critical technique used in pharmaceuticals, biotech, and diagnostics to preserve biological materials.
The Freeze-Drying Process in Four Stages
Pretreatment (Formulation)
Before freeze-drying begins, specific stabilizers or additives may be introduced to maintain product integrity and improve efficiency. In pharmaceuticals, this step ensures the preservation of proteins, enzymes, or reagents.
Freezing Stage
The product is cooled below its triple point, a temperature where solids, liquids, and gases coexist. This helps create an optimal ice crystal structure, which is critical for the efficiency of sublimation in later stages.
Primary Drying (Sublimation)
Under a vacuum, controlled heat is applied to convert ice directly into vapor, removing up to 90% of moisture without damaging delicate biological materials. This is the core of the freeze-drying process.
Secondary Drying (Desorption)
To eliminate any remaining moisture, a second drying phase applies higher vacuum pressure and heat, ensuring long-term stability. Finally, the product is sealed with sterile air or inert gas for storage.
Why Freeze-Dry?
In medical applications, freeze-drying extends the shelf life of reagents and vaccines, preventing degradation caused by moisture. Similarly, in coffee production, lyophilization preserves flavor and aroma, making it possible to store instant coffee for long periods.
This process is a key innovation in drug development, ensuring that biological components remain stable while allowing for easy rehydration when needed—whether for diagnostic assays or simply a cup of coffee.
Join Nick and Joris in this engaging discussion as they uncover the science behind lyophilization and why it’s crucial in both medical and everyday applications.
The Science of Freezer-Drying
Related Resources

From how much of your body is actually bacterial to how fast microbes can multiply, these facts are designed to stick with you long after the party ends.
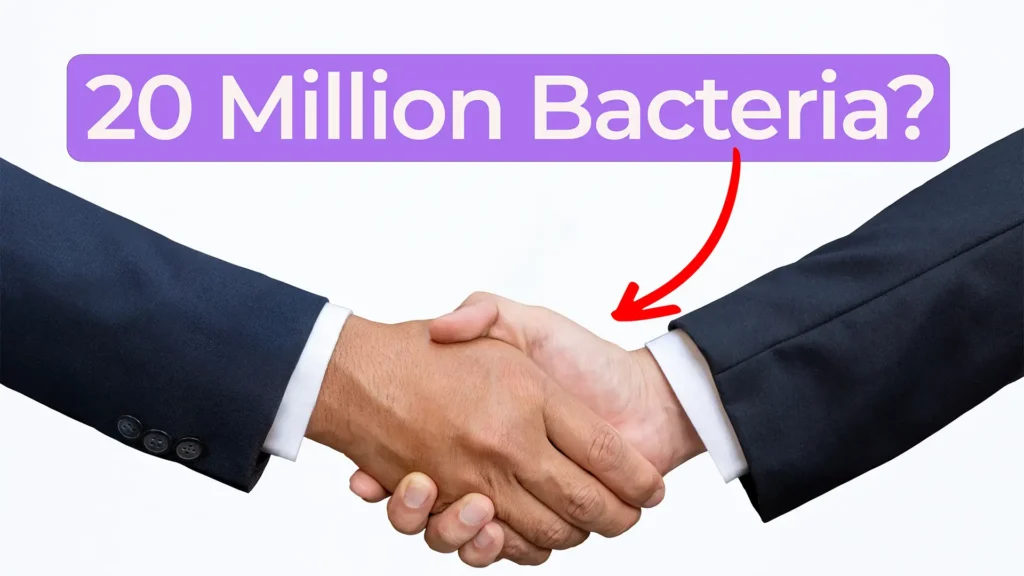
In this Bio Break episode, Nick and Nigel explore a surprising and memorable microbiology fact that puts everyday hand hygiene into perspective.
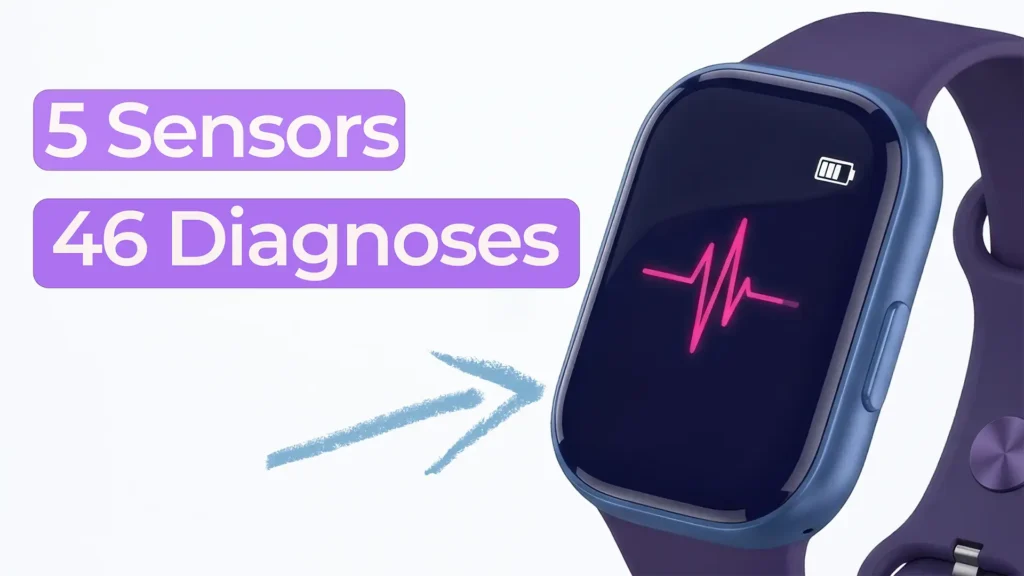
Nick and Nigel explore how a surprisingly small set of sensors could be used to identify a wide range of common health conditions.

Nick walks through a practical Teflon tape lesson that came from real work supporting a mechanical test rig.
