
Bio Break: How to Successfully Pivot in Medical Device Development
In the world of medical device development, unexpected challenges often lead to critical product pivots. In this episode of Bio Break, Nick and Joris discuss one of the most dramatic pivots they’ve encountered—transforming a lab-developed test (LDT) into a lateral flow assay to expand its market reach.
Initially, the client had a blood-based diagnostic test licensed for use in North America but wanted to serve global markets. Their original approach involved developing a blood spot sampling system, where patients would send dried blood samples by mail for lab analysis. However, after evaluating the logistics, the team proposed a game-changing pivot: converting the assay into a lateral flow test, making it more accessible and reducing the need for centralized lab processing.
This shift represented a major transformation—moving from a laboratory test requiring trained technicians to a rapid test that could be performed by users at home. This meant rethinking regulatory strategies, usability considerations, and product stability while maintaining diagnostic accuracy. The outcome? A scalable and commercially viable point-of-care diagnostic that opened new global opportunities for the company.
Pivoting in medical device innovation isn’t just about solving technical challenges—it’s about recognizing when to rethink market access, user needs, and business models. In this conversation, Nick and Joris highlight the key considerations when making strategic pivots and the lessons learned from adapting diagnostic technologies to new formats.
How to Successfully Pivot in Medical Device Development
Related Resources

From how much of your body is actually bacterial to how fast microbes can multiply, these facts are designed to stick with you long after the party ends.
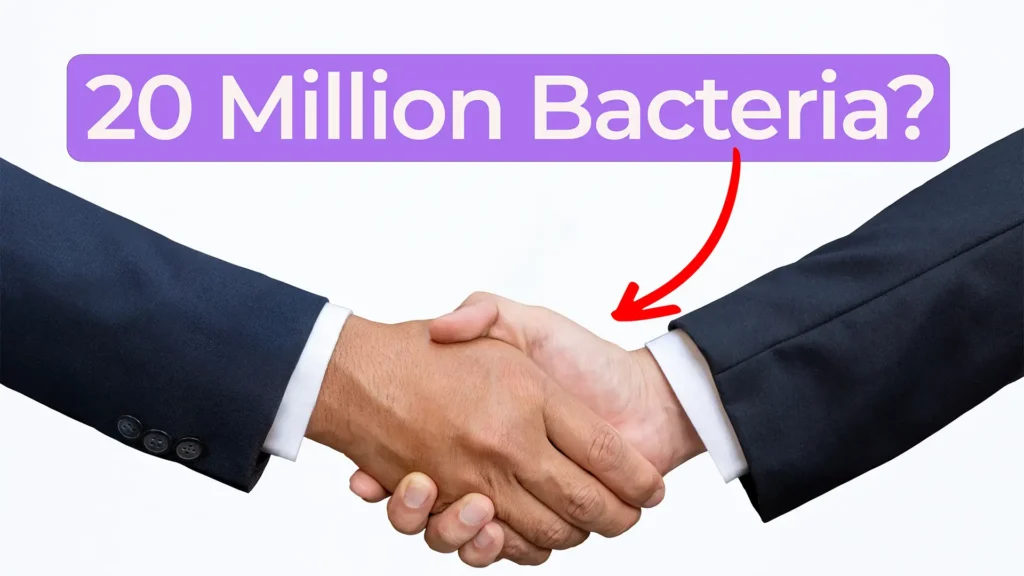
In this Bio Break episode, Nick and Nigel explore a surprising and memorable microbiology fact that puts everyday hand hygiene into perspective.
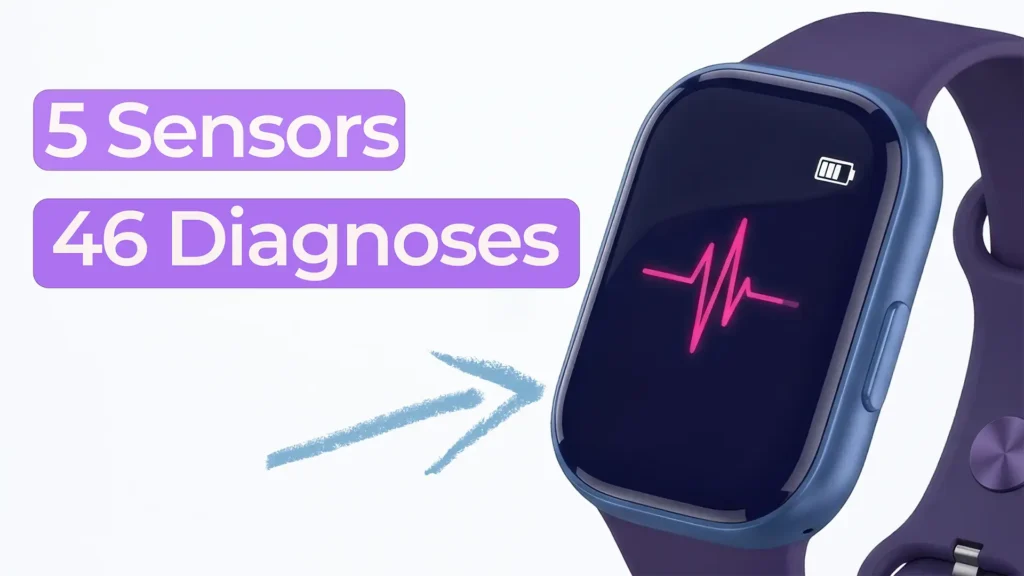
Nick and Nigel explore how a surprisingly small set of sensors could be used to identify a wide range of common health conditions.

Nick walks through a practical Teflon tape lesson that came from real work supporting a mechanical test rig.
