
Tissue Simulation: Advances for Next-Gen Medical Devices
Introduction
Today, 85% of the top 50 healthcare companies use Computational Modeling and Simulation (CM&S) to develop their products and processes [1]. Whether it’s refining overall device parameters or optimizing critical requirements, engineering simulations help reduce development timelines and enhance design exploration.
Innovation and speed to market are paramount in an increasingly competitive MedTech landscape, where patentable technologies can directly impact patient care and clinical outcomes. In this context, tissue modeling has become a pivotal simulation tool in preclinical device development. These models replicate the complex interactions between mechanical forces and thermal effects within human tissues—an essential capability when evaluating devices that interact with or influence biological systems.
This blog explores three key aspects of tissue modeling: (1) technical context, (2) why tissue modeling matters, and (3) its impact on medical device programs.
Technical Aspects of Mechanical-Thermal Tissue Models
Fundamentals of Tissue Modeling
Human tissues exhibit complex, nonlinear mechanical properties—including anisotropy, viscoelasticity, and hyperelasticity. When exposed to external forces or thermal effects, their responses are intrinsically coupled. For example, when a device generates heat—whether for tissue ablation in cancer therapy or localized hyperthermia—tissue properties can change through thermal expansion, altered stiffness, or phase transitions (i.e. varying water content). Accurate simulation of these responses can be critical for effective device design.
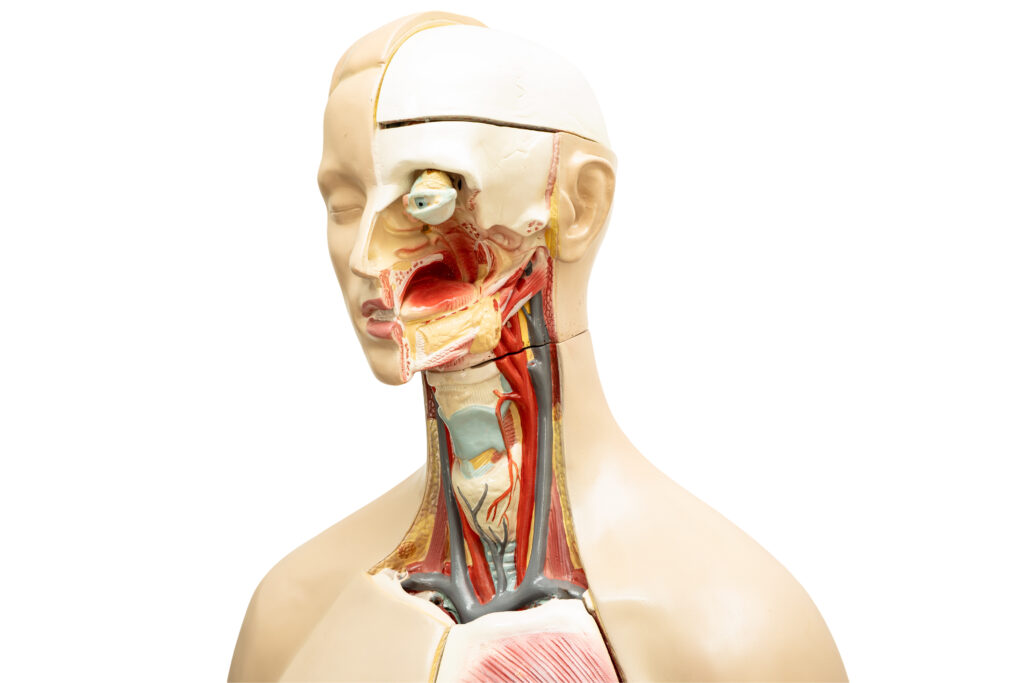
Coupled Physics and Material Models
Tissue simulations often leverage finite element analysis (FEA) to break down biological structures into manageable elements. This enables integrated modeling of mechanical and thermal responses. Multiphysics simulations typically combine continuum mechanics with bioheat transfer models, such as Pennes’ bioheat equation [2], to capture heat distribution and its impact on tissue deformation.
Material properties for simulations can be derived from both literature and experimental data (from in vivo or ex vivo studies), including thermal conductivity, specific heat, elastic modulus, and viscoelastic damping. Advanced computational techniques manage the dynamic interplay between thermal gradients and mechanical stress, employing both explicit and implicit integration schemes depending on model complexity.
Sophisticated constitutive models are required to capture nonlinear tissue behavior. Hyperelastic models—such as Mooney-Rivlin or Ogden [3]—are commonly used to replicate large deformations in soft tissue, often combined with viscoelastic formulations to model time-dependent responses [4]. When thermal effects are included, constitutive equations incorporate temperature-dependent terms to capture thermoelastic behavior. As tissues heat, stiffness may decrease, plasticity may increase, and tissue coagulation may occur—all of which the simulation must dynamically track.
Validating and Calibrating Tissue Simulations
When simulations inform safety or efficacy decisions, rigorous validation is essential. Tissue models are often benchmarked against experimental setups using tissue samples or surrogates like hydrogels. Techniques such as tensile/compression testing, atomic force microscopy, pipette aspiration, and dynamic elastography [5] characterize mechanical properties, while infrared thermography and digital image correlation (DIC) map temperature gradients and deformation.
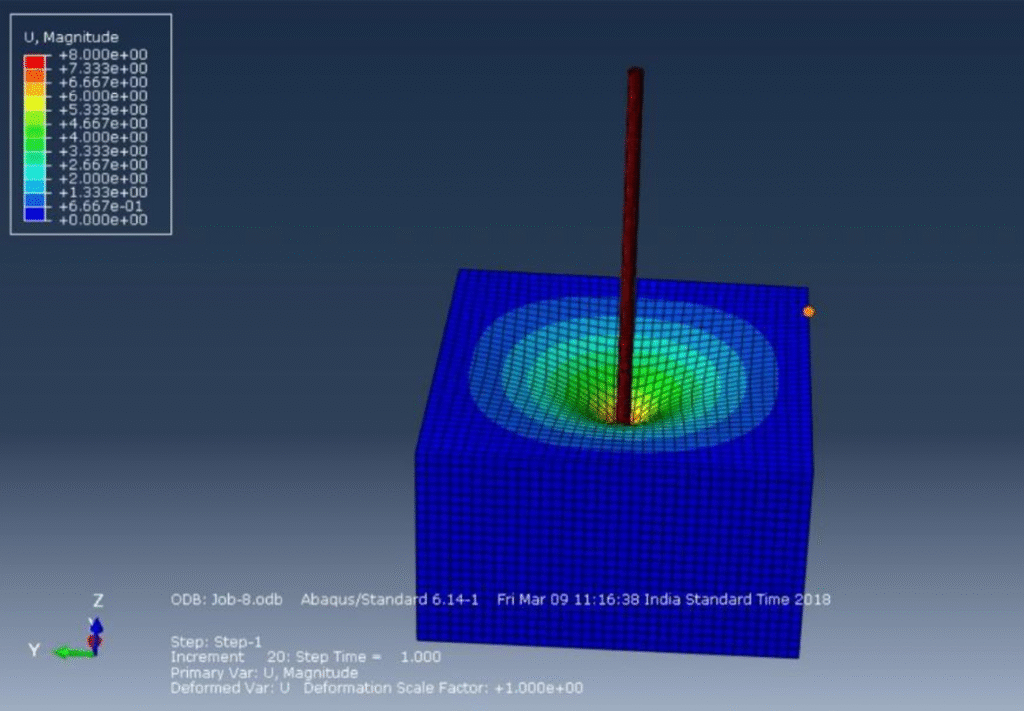
Many tissue interaction models are phenomenological rather than purely mechanistic [4]. Experimental data helps fine-tune models to ensure their predictive accuracy and relevance within the validated parameter space for subsequent device testing.
The Importance of these Models
How Tissue Modeling Enhances Device Safety
Tissue modeling delivers powerful predictive capabilities, allowing engineers to anticipate device-tissue interactions before clinical testing. This is especially critical for energy-delivery devices—such as radiofrequency ablation systems, high-intensity focused ultrasound (HIFU), or laser-based surgical tools—where unintended thermal injury could have serious consequences. Simulations can also identify “hot spots” and regions of mechanical and thermal-induced stress not easily captured in physical bench tests, enabling early design adjustments that enhance both device safety and human factors engineering outcomes. Additionally, simulations help bridge in vitro and in vivo testing, reducing reliance on costly, ethically complex animal studies while accelerating development cycles. Also, regulatory agencies increasingly accept and promote the use of validated simulation data as part of regulatory submissions, which can streamline approval processes and demonstrate device performance under thermal and mechanical stresses.
Economic Benefits and Expanding Clinical Applications
Simulation platforms offer substantial economic benefits by reducing prototyping costs, shortening development timelines, and supporting more efficient iteration. They also enable simulation of patient-specific conditions by incorporating anatomical variability—for example, modeling a range of fat and muscle thicknesses to identify worst-case heating scenarios in implants. This is especially valuable for complex anatomical regions like joints, where first-principles calculations are insufficient.
Mechanical-thermal modeling supports a wide range of emerging medical devices, from wearable therapeutics to implantable drug delivery systems. These insights also inform surgical planning and procedural training, enhancing clinical decision-making and treatment outcomes.
A 2023 case study presented at the Design of Medical Devices Conference showcased finite element modeling of a transcatheter aortic valve (TAV) and its interface with the aortic root. The model was qualified under the FDA-recognized ASME V&V 40 risk-based framework and directly contributed to device clearance—setting a new precedent for regulatory submissions. Today, FEA models of TAVs and surrounding tissues routinely replace large portions of bench fatigue testing, offering a scalable strategy for applying CM&S across diverse device applications.
Future Directions for Medical Device Development Programs
Personalized Modeling and Real-Time Simulation
The next frontier for these tools lies in integrating patient-specific data—such as CT or MRI imaging—into personalized tissue models. This enables clinicians to tailor therapies like thermal ablation, such as integrating patient-specific tissue conductivity and perfusion rates, enabling clinicians to tailor energy delivery precisely during surgical planning stages, minimizing collateral damage while maximizing therapeutic outcomes. Such individualization aligns with the broader shift toward personalized medicine.
Advancements in Multiphysics and Cross-Disciplinary Collaboration
As computational power and multiphysics software evolve, mechanical-thermal tissue models will achieve even higher fidelity. Enhanced meshing, advanced solvers, and machine learning integration will enable faster, more accurate simulations and real-time design feedback, all with less computational resources. Tissue models are inherently multidisciplinary, drawing on biomechanics, thermal physics, materials science, and computational modeling. Collaborative efforts between engineers, clinicians, and CM&S specialists are driving innovation, with ongoing research exploring the addition of biochemical responses to thermal interactions—expanding the clinical relevance of these simulations.
Integrating Simulations into Regulatory Pathways
For developers and regulatory professionals, understanding the quality of simulations is crucial, especially where patient risk is involved. Simulation evidence is now a standard component of many regulatory submissions, with frameworks like ASME V&V 40 [6] providing clear guidance (codeveloped by the FDA). Engaging early with regulators and incorporating validated simulations into formal verification processes helps ensure that devices meet stringent safety and performance requirements.
Conclusion
Mechanical-thermal human tissue models represent a transformative approach to medical device development, blending advanced engineering with biological realism. These simulations enhance device safety, optimize design, and accelerate R&D, enabling a culture of evidence-driven innovation.
As simulation tools continue to advance, their predictive accuracy and clinical applicability will expand—empowering the next generation of devices designed for the dynamic realities of human tissues in representative environments. This paradigm shift is poised to redefine safety and performance standards in medtech, with predictive modeling driving future clinical success and patient outcomes.
TL;DR / Key Takeaways
- Tissue modeling improves predictive accuracy and patient safety for energy-delivery and implantable devices.
- Coupled mechanical-thermal simulations optimize device design and reduce reliance on animal studies.
- Validated simulations support regulatory submissions, accelerating approval timelines.
- Personalized modeling enables precision therapies tailored to individual anatomies.
- Advances in software and machine learning are driving the future of real-time, high-fidelity simulations.
Nathan Muller is a StarFish Medical Mechanical Engineer – Analysis and Design. His focus is in simulation engineering using computational modelling. As part of a design and development team, he frequently leads the development of mechanical design and device integration across disciplines, including targeted optimization and derisking activities through computational modelling and simulation (CM&S).
References
[1] Boston Engineering, “Disruption in Healthcare Is Here: Are You Ready?,” December 2021. [Online]. Available: https://www.boston-engineering.com/wp-content/uploads/2021/12/Healthcare-White-Paper-Disruption-in-Healthcare-is-Here-Are-you-Ready-2020-New-min2.pdf. [Accessed 16 May 2025].
[2] K. Navindaran, J. S. Kang and K. Moon, “Techniques for characterizing mechanical properties of soft tissues,” Journal of the Mechanical Behaviour of Biomedical Materials, vol. 138, 2023.
[3] C. R. Ethier and C. A. Simmons, Introductory Biomechanics: From Cells to Organisms, Cambridge University Press, 2007.
[4] E. H. Wissler, “Pennes’ 1948 paper revisited,” Journal of Applied Physiology, pp. 35-41, July 1998.
[5] ASME, “ASME V&V 40,” 2018. [Online]. Available: https://www.asme.org/codes-standards/find-codes-standards/assessing-credibility-of-computational-modeling-through-verification-and-validation-application-to-medical-devices.
[6] K. K. Dwivedi, P. Lakhani, S. Kumar and N. Kumar, “A hyperelastic model to capture the mechanical behaviour and histological aspects of the soft tissues,” Journal of the Mechanical Behavior of Biomedical Materials, 2021.
Related Resources

Ariana Wilson and Mark Drlik break down medical vs wellness devices and explain why two products with identical hardware can fall into completely different regulatory categories.

Nick and Nigel break down the ELISA assay explained in simple, practical terms using everyday models.

Ariana Wilson sits down with Mark Drlik to unpack why reprocessing is often one of the hardest challenges engineers face during development.

Nick and Nigel walk through how teams decide between ethylene oxide, E-beam, and gamma radiation sterilization.