The importance of a user-centered design approach to training: A case study
The simplest and least expensive way to train users of medical devices is to ask them study the Instructions for Use (IFU) beforehand. However, reliance on end users to thoroughly read and understand the IFU is often unrealistic and may be insufficient for complex devices or devices where use errors may result in serious harm to the user or patient.
Unlike everyday appliances where trial and error might suffice, medical devices demand precise operation to ensure their safe and effective use. Not only are use errors a potential safety concern but they may also result in regulatory submission delays and potential costly redesign. In this case study, we will demonstrate how adopting a user-centered design approach to training can significantly minimize potential use errors, enhancing both safety and efficiency.
A few years ago, I was involved in a usability study for a new surgical device. The surgeons recruited as subjects for the study were provided with the IFU days in advance of the usability testing session and were instructed to read and understand the IFU as this was the only training they would be receiving for the device. It was understood that this training approach was unlikely to be followed in a real-world scenario, but we were testing a plausible, worst-case scenario in this usability test. We estimated about 75% compliance, but to our surprise, none of the surgeons had read the IFU prior to the session. Ideally, we would have excluded all subjects who had not read the IFU, but with zero compliance it was impossible to make up the shortfall with alternate subjects so testing proceeded with the untrained subjects. As expected, the lack of training resulted in significant usability findings in our testing.
The above example illustrates that relying on users to read the IFU may be the least expensive training option, but it may be insufficient on its own. Even if you implement a competence check, such as a quiz, to ensure users have read the IFU, this only confirms they have looked at the IFU, it does not guarantee they have learned the necessary skills to operate the device safely and effectively, so additional training approaches should be considered. Users can be trained with a variety of methods, including:
- Self-directed training: Reviewing the Instructions for Use or quick reference guide (with or without a quiz)
- Didactic training session: In-person classroom or video sessions
- Hands-on training: Group or individual hands-on training sessions using a suitable patient analogue and environment
By identifying and analysing the skills and/or knowledge that are most important for users to acquire to operate the device safely and effectively, you can better match your users’ needs to the training approaches adopted. Once a training program is developed, it is then vital to test that the training program is sufficient at transferring the necessary skills and reducing use errors.
To illustrate this, let us revisit our user study again. The results from our earlier testing highlighted the insufficiency of self-directed training with the IFU alone and reinforced the recognition of the complex nature of certain aspects of the device’s usage. This led to the development of an enhanced training strategy. In addition to IFU review, users were provided with training videos and were required to attend an instructor-led classroom and hands-on training sessions for the proposed surgical device.
To test the effectiveness of this training approach, we conducted a usability testing session where all users underwent the updated training program. As expected, the number of use errors decreased compared to our initial testing, where none of the users had read the IFU. However, some use errors persisted during stages of the surgical procedure. Test subjects were interviewed about which aspects of the training or the device’s user interface influenced their actions or inactions. It turned out that issues arose in parts of the procedure where users had not had hands-on experience during training. The training was conducted in a group setting, with one surgeon performing a step while the others watched. Although each surgeon performed a step in the procedure, they did not handle the device for every step. Some users admitted that they were not paying full attention to the steps they were not performing, especially towards the end of the training session when attention spans naturally wane.
Why did some users still fail to retain key knowledge despite the new, comprehensive training strategy? The answer is multifactorial, but a key aspect is understanding how your users best learn to use the device. Learning styles can be categorized using frameworks like VARK or Kolb’s learning styles. Published data may indicate the preferred learning style for specific user groups, but this data might not always be available or applicable to your medical device. Always ask your users which methods they find most effective for learning to use new medical devices. For example, surgeons often prefer hands-on learning (1,2), although one study suggests that specializations like neurosurgeons may favor reflective observation (3). In our case study, we found that users needed to physically interact with the device for all steps of the surgical procedure to ensure effective training and recall. Consequently, we revised our training strategy to allow all surgeons to perform each step of the procedure, effectively eliminating use errors stemming from ineffective training or inattention. While this approach may not be necessary or suitable for all applications, it should be considered for complex devices or those where serious harm could occur. Determining an effective training strategy for this case study was made possible through frequent usability testing with actual users.
In addition to tailoring your training strategy to your users’ preferred learning style, training recall can be enhanced by ensuring the device is designed intuitively using user-centered design principles, including visual cues to guide users (accommodations/signifiers) (4). For some medical devices, it is beneficial to reduce reliance on semantic memory (facts) and leverage accommodations and signifiers to minimize the need for extensive training or instruction. This approach is particularly useful for devices that are infrequently used or used in high-stress environments like emergency rooms, where reliance on cognitive memory should be avoided.
When deciding how users will learn to use the device, consider the following steps:
- Consider the device/environment: Is the device complex? Could use errors result in serious harm? Is the device used infrequently? Will it be used in a high stress environment?
- Use user-centered design principles: Make the design as intuitive as possible (accommodations/signifiers) to reduce training burden and cognitive load.
- Define a training strategy: Based on the above, decide on the appropriate training method (IFU/QRG, didactic, hands-on training).
- Test your training strategy.
- Iterate based on findings, if needed.
For complex devices or those where use errors could result in serious harm, ensure you address how users will best learn to use the device and test your training strategy well before final validation. By taking this approach, you can minimize use errors during validation testing and post-marketing safety findings, ultimately designing a safe and effective medical device.
TL;DR
- IFUs alone are insufficient for training on complex or high-risk medical devices.
- A user-centered training approach reduces use errors by aligning with actual user behavior and learning preferences.
- Usability testing revealed that passive training (watching or reading) leads to critical gaps in skill retention.
- Training should consider hands-on and scenario-representative approaches, especially for complex devices or those where serious harm could occur.
- Incorporating learning style frameworks (e.g., VARK, Kolb) and continuous feedback can drive better training design and safer outcomes.
Paul Hulme is a StarFish Medical Human Factors Engineer. His professional experience includes working with the Canadian Space Agency and 5 years in Switzerland working at Zimmer GmbH. Paul studied Mechanical Engineering at the University of Victoria, completed his Masters of Mechanical Engineering at the University of Calgary, and his PhD in Biomedical Engineering at the University of Bern.
Images: Adobe Stock
References
1: Engels PT, de Gara C. Learning styles of medical students, general surgery residents, and general surgeons: implications for surgical education. BMC Med Educ. 2010 Jun 30;10:51. doi: 10.1186/1472-6920-10-51. PMID: 20591159; PMCID: PMC2909974.
2: Shanmuganathan K, Liang K, Vignaraja V, Galloway R, Chandrakumar C. The learning preferences of aspiring orthopaedic surgeons in the UK. Br J Hosp Med (Lond). 2023 Feb 2;84(2):1-12. doi: 10.12968/hmed.2022.0461. Epub 2023 Feb 23. PMID: 36848161.
3: Lai HY, Lee CY, Chiu A, Lee ST. The Preferred Learning Styles of Neurosurgeons, Neurosurgery Residents, and Neurology Residents: Implications in the Neurosurgical Field. World Neurosurg. 2014 Sep-Oct;82(3-4):298-303. doi:10.1016/j.wneu.2014.04.067. Epub 2014 May 2. PMID: 24793980.
4. Masoudi, Nafiseh (1); Fadel, Georges M. (1); Pagano, Christopher C. (2); Elena, Maria Vittoria (1). A review of affordances and affordance-based design to address usability. International Conference On Engineering Design, ICED19 5-8 August 2019, Delft, The Netherlands
Related Resources

Ariana Wilson and Mark Drlik break down medical vs wellness devices and explain why two products with identical hardware can fall into completely different regulatory categories.

StarFish Medical exhibits at MD&M West 2026, showcasing multidisciplinary medical device design, development and commercialization expertise.

Nick and Nigel break down the ELISA assay explained in simple, practical terms using everyday models.
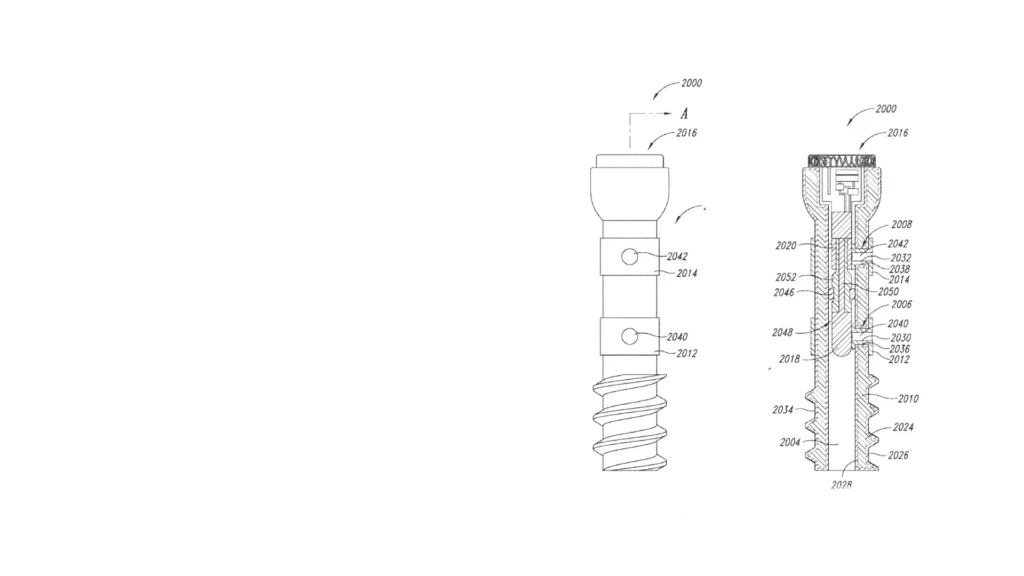
StarFish Medical helped Canary Medical develop a smart implantable screw using EIS to monitor fracture healing without repeated imaging.