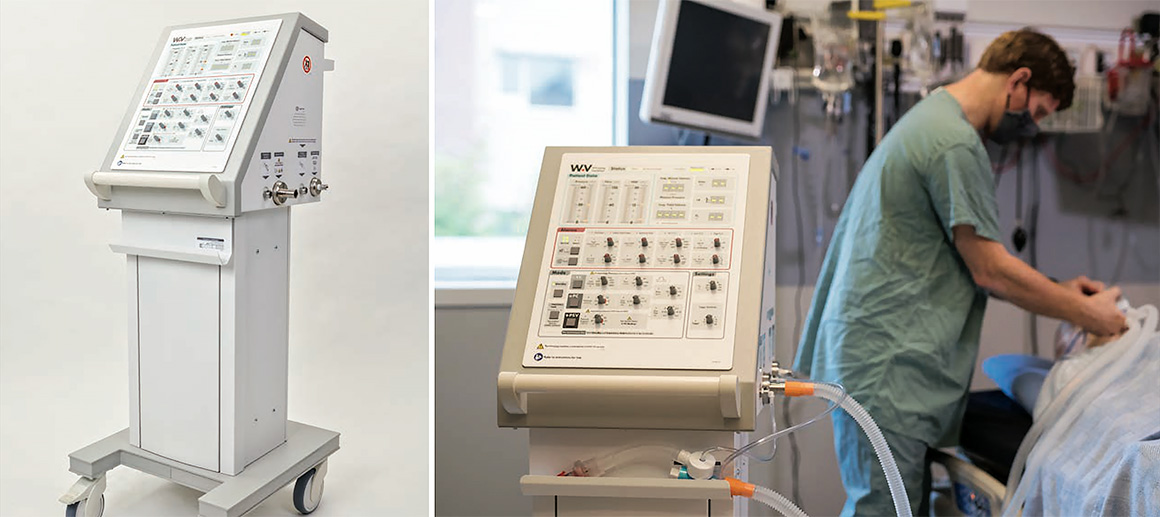
Winnipeg Ventilator 2.0
When the Government of Canada appealed to the med tech industry for emergency pandemic ventilators at the arrival of the COVID-19 pandemic, StarFish Medical signed on to lead a consortium that would create a Ventilator 2.0 therapy device in record time.
The Challenge
An immense design and engineering effort was needed to produce a Health Canada approved ventilator in just 6 months, a project that would have taken up to 10 years under normal circumstances. Design constraints were quickly defined in order to speed development. The Ventilator 2.0 would be based on a proven, effective platform, yet easy to use and manufacture in developed and developing countries alike. The Winnipeg Ventilator 2.0 is based on the pioneering work of Dr. Magdy Younes.
The Process
Design for pandemic supply chains and use conditions was crucial. Minimal reliance on commonly used ventilator components meant a physical rotary knob and LED user interface with a piston pump instead of a typical turbine design ventilator.
Ergonomic data, user feedback, and user testing results guided the design and positioning of key user touchpoints such as the gas ports, push handles, and control panel. Unlike most other emergency pandemic ventilators, the Winnipeg Ventilator 2.0 offers a full suite of ventilation modes needed to help an ARDS patient fully recover.
Results
StarFish Medical designed a Health Canada certified ventilator in 6 months that is easy to use, manufacture, and accessible to both developed and developing nations alike.
Bottom Line
The Winnipeg Ventilator 2.0 has been authorized for use by Health Canada under the Interim Order Respecting the Importation and Sale of Medical Devices for Use in Relation to COVID-19.