This blog describes a novel approach to develop two systems – an aerosol drug delivery system prototype and a system to emulate physiological kinetics/read aerosol measurement through laser diffraction. The project’s success depended on replicating the intricate conditions of the human respiratory system, fine-tuning formulations for preferred aerosol particle size for drug delivery to the pulmonary region of the airway, and creating a handheld working prototype for integration demonstration and investor presentation.
Aerosol-based pulmonary-delivered drug devices offer significant value by enabling targeted drug delivery directly to the respiratory system, enhancing treatment efficacy while minimizing systemic side effects. In today’s personalized healthcare landscape, precision drug delivery systems are crucial in ensuring both efficacy and patient safety.
Benchtop Model of the Human Airway
The first phase of the project involved designing a benchtop heated and humidified human airway model, simulating anatomical-like conditions of drug delivery to the pulmonary region. This model replicated the cross-sectional area and transitions (e.g., tracheal diversion), thermal, and humidification characteristics found in human respiratory tracts, providing an accurate environment for evaluating aerosol behavior.
Aerosol is generated initially through compressed gas pressure via a spray nozzle and modified through a segmented heated pathway. As the aerosol transits through the airway regions, smaller aerosol particles grow due to high relative humidity and combination with other particles, while larger particles impacted walls. The laser pathway read area at the pulmonary region of the airway reviewed both the volume of aerosol and their sizes at the critical retention region where drugs are intended to be delivered.
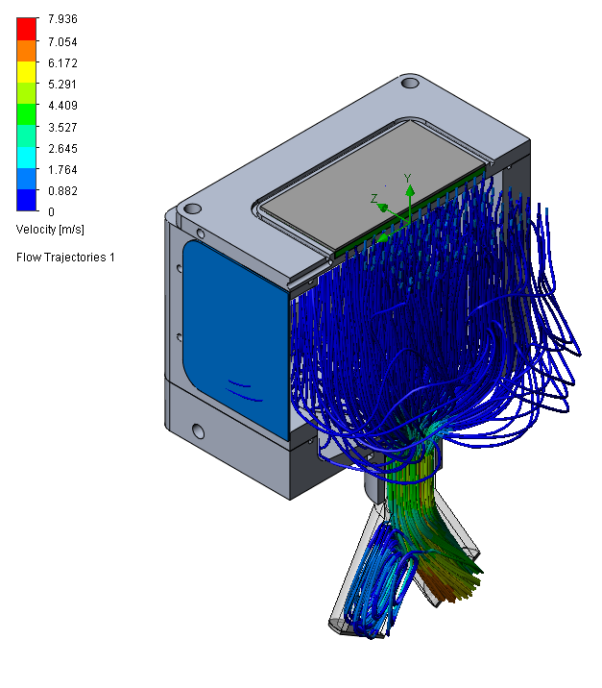
The initial prototype had an issue with eddy currents across the laser light pathway, causing noise around higher values for the retained aerosol sizes that were caught in the current. To optimize the flow, a design study using computational fluid dynamics showed great improvement in the flow pathway. See the vector plot comparison in Figure 2.
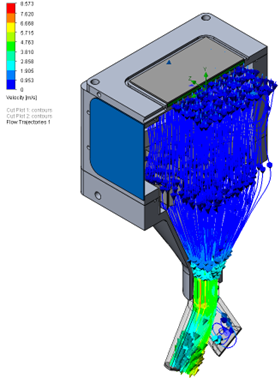
Constructing a reliable and physiologically accurate airway model provided a testing platform that could evaluate drug delivery performance under real-life conditions. This enabled critical insights into how the product would produce aerosol and transit through the human body, significantly reducing the risk of later-stage development integration.
Join over 6000 medical device professionals who receive our engineering, regulatory and commercialization insights and tips every month.
Precision Particle Sizing with Laser Diffraction
Unlike oral or intravenous medications, which must pass through various biological barriers and can face degradation or reduced uptake, aerosol-based delivery provides a more direct route by allowing drugs to reach the pulmonary region of the lung where they can be absorbed directly into the bloodstream. By controlling aerosol particle size, these devices can optimize drug deposition in the airway, ensuring that the medication reaches its intended target while minimizing waste along the transit route.
Another crucial element of this solution is the integration of laser technology to measure aerosol particle size in real time. Drug delivery effectiveness in the lower airway is highly dependent on the size of aerosol particles, as larger particles may deposit in the upper respiratory tract while particles that are too small may be exhaled before they reach their target.
As shown in Figure 3, aerosol around 0.05-1um transit through the airway without being deposited, while those >1um tend to deposit in the pulmonary region (lower lung). Aerosol tends to gain in size through transit via agglomeration and through absorbing surrounding humidity, which allows initially small aerosol to transit through the upper airway without impaction, growing along the way, and be deposited in the lower airway. The airway model we developed allowed for size readings of aerosol in the lower lung region of the system.
Figure 4 shows a representation of the range of particle sizes in this region, providing the right range of particle sizes that could be retained in the pulmonary region.
Laser-based particle sizing provided accurate, real-time data allowing for finely tuned particle size distribution vs. other factors. This optimized drug delivery to the lower lung, building confidence in the drug formulation. This capability represents a substantial value proposition – it showcases not just a working device but one that could deliver particles in the optimal size range for enhanced efficacy, which is a critical aspect for gaining market acceptance.
Flexible Aerosol Testing Platform
Next, we developed an open-frame aerosol generation system which included a heated chamber and a metered dose dispensing mechanism. This system enabled the creation and delivery of precisely controlled aerosolized drugs under laboratory conditions.
Prototype Creation and Product Embodiment

Once the benchtop system demonstrated successful results, the next step was to translate those findings into a market-ready device. We designed and built a prototype handheld drug delivery system that met the client’s product vision, while showcasing the optimal aerosol performance.
The prototype wasn’t just about technical integration; it enabled the client to market their innovation effectively, translating the product vision to stakeholders and potential investors. It provided proof of concept, showing that the technology wasn’t just theoretical – it was ready for real-world application.
Conclusion: Comprehensive Value Delivered
Aerosol drug delivery systems demonstrate immense value by providing targeted drug delivery directly to the respiratory system. This approach enhances treatment effectiveness while reducing systemic side effects, making it a vital tool in the era of personalized healthcare. The successful development of both an aerosolized drug delivery prototype and a system to replicate physiological conditions for aerosol measurement using laser diffraction highlights the importance of precision. By replicating the complex environment of the human respiratory system, optimizing formulations for ideal particle size for pulmonary delivery, and creating a functional handheld prototype, this project not only achieved its technical goals but also laid the foundation for future integration and investment opportunities.
For more information, please contact Michael Breede at 203-403-7331 or minskoffbreede@yahoo.com.
Nathan Müller is a StarFish Medical Mechanical Engineer – Analysis and Design. His focus is in simulation engineering using computational modelling. As part of a design and development team, he frequently lead the development of mechanical design and device integration across disciplines, including targeted optimization and derisking activities through computational modelling and simulation (CM&S).
Images: StarFish Medical
Read our summary of the FDA Guidance Document “Assessing the Credibility of Computational Modeling and Simulation in Medical Device Submissions (17th November 2023)”