Developing innovative and life-changing products provides a wellspring of inspiration as our “destinations.” But “anything worth doing is never easy” and this is a pretty accurate capture of medical device product development. If we are going to spend most of our time on the journey, wouldn’t it be a whole lot more enjoyable if we have trust in and feel comfortable with our travel companions?
The reality is that when we invest in our relationships and appreciate the journey, we are much more likely to reach our goals. Ironically, focusing too much and rushing towards the destination may be the single biggest obstacle to getting there.
This blog examines six common destinations in medical device development with an overview of our High Level Program Planning (HLPP) approach to medical device journey (development), focusing on the important steps and conversations that tee up a well-positioned and aligned program plan.
Common Medical Device Development “Destinations”
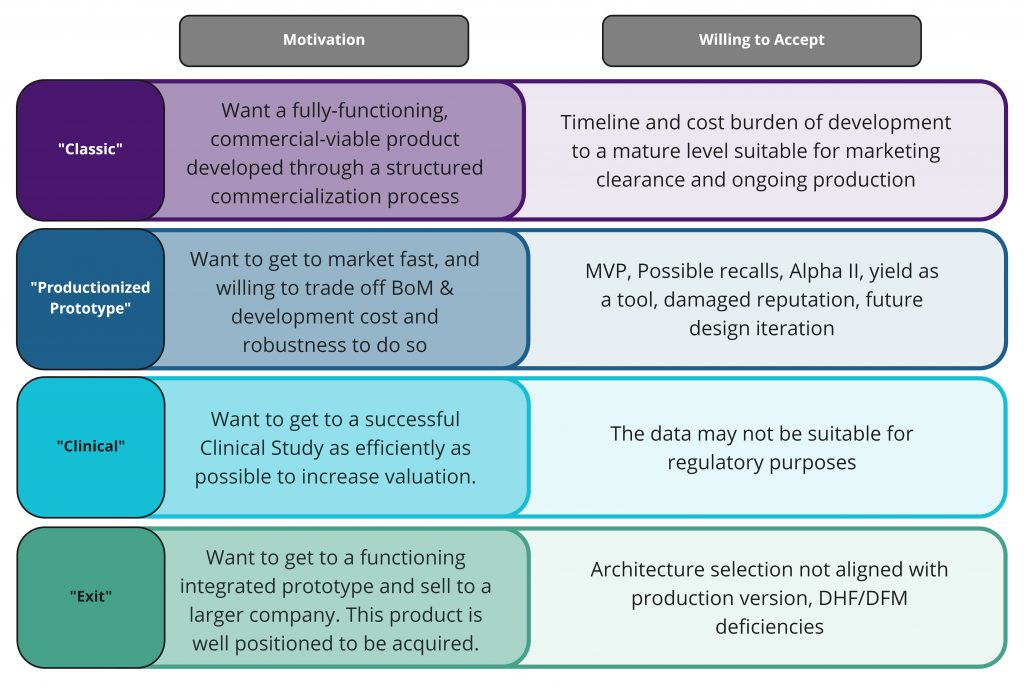
- Small start-up with an idea, seeking investors to fund their project. Looking for: Investor collateral – ID sketches / renderings, block diagram, HLPP.
- Company and funding is dependent on evidence that their idea is viable. We have exciting technology. Looking for: Benchtop Demo, Technical de-risking, Pathfinder Product Definition.
- Get to a functioning integrated PoC and sell to a larger company. This product is well positioned to be successfully acquired. Looking for: Demonstration PoC, Regulatory Pathway/collateral, IP.
- Want to get to a successful Clinical Study as efficiently as possible to increase valuation. Looking for: Successful In Vivo results for the core technology.
- Want to get to market fast, and willing to trade off BoM & development cost and robustness to do so. Looking for: Minimally Viable Product that is commercially saleable.
- Mature company with the program funding and experience to take their product to market. Looking for: a fully-functioning, commercially-viable product, developed through a structured commercialization process.
Each of these destinations requires a tailored program strategy to navigate technology development, product definition, product development, and new product introduction phases as appliable. To support clients in mapping out the journey, we use a staged High Level Program Plan process.
For the quick hits, here are 3 takeaways to keep in mind about High Level Program Plan development:
- High Level Program Planning is a collaborative journey and ongoing discussion with the key stakeholders/client.
- The foundation of a meaningful Program Plan is well understood key stakeholder/client business objectives and success milestones.
- Leverage Program Type segmentation and historical comparable program data to build tailored program plans instead of bottoms up estimates.
The High Level Program Plan development process incorporates five stages with an emphasis on key stakeholder/client engagement throughout, Program Type segmentation for tailoring, and historical project data as a basis for timeline and labour projections:
- Stage Zero: Program Strategy and Alignment – Leverage Program Type segmentation to explore program trade-offs and dig deeper to understand the key stakeholder/client business objectives and success milestones.
- Stage One: Scoping – Understand the system architecture and design definition. Identify comparable historical programs based on product development roadmap and system architecture with support from Functional Managers and SF team members with history.
- Stage Two: Estimating Schedule and Labour – Mine historical program actual timelines and labour spend, then scale based on similarities and differences to arrive at nominal program estimate. Crosscut the assessment based on project team size and estimated program duration range to generate an alternative viewpoint estimate from full time equivalent employee burn rates.
- Stage Three: Reviewing and Tailoring – Review schedule with the key stakeholders/client. Review cost estimates with Functional Managers.
- Stage Four: Construct and Present HLPP – Construct the HLPP Deliverable, incorporate the approved labour and material estimates, and present to the key stakeholders/client. If we’ve done our jobs, the final deliverable and cost projections shouldn’t come as a surprise, and the plan should be well aligned to client business goals.
Going forward the HLPP serves as a baseline that we – as a collective team with the client – use to build detailed statements of work and measure deviations in the plan.
How Much Does it Cost to Develop a Medical Device?
Image: StarFish Medical
Julian Grove is a Sr. Systems Engineer in Product Development at StarFish Medical. He earned his Bachelor of Engineering (BEng) degree in Mechanical Engineering from the University of Victoria and spent several years developing mechanical solutions for medical devices with StarFish while maintaining an eye for the overall system design.
Mark Drlik is the Director of Program Design in Business Development at StarFish Medical. With over 20 years of product development experience, he works with clients to transition medical devices from initial product architecture definition to final product delivery to ensure client success.
Discover the Benefits of Enterprise Partnerships and Working with StarFish Medical.