Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Ariana Wilson and Mark Drlik break down medical vs wellness devices and explain why two products with identical hardware can fall into completely different regulatory categories.
-

Ariana Wilson sits down with Mark Drlik to unpack why reprocessing is often one of the hardest challenges engineers face during development.
-

Understanding how clinical ventilator development differs from commercial ventilator design is essential for teams planning early studies.
-

When Ariana Wilson and Mark Drlik take apart a common appliance, they uncover engineering principles that connect directly to medtech.
-
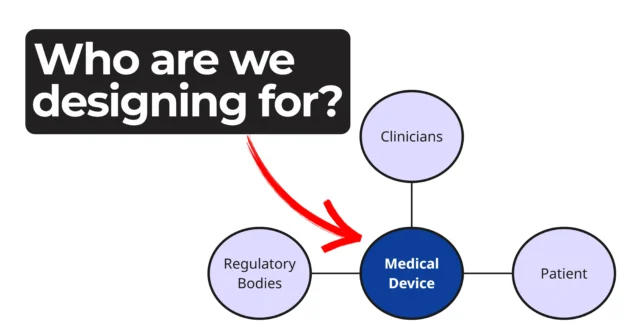
Every phase of a device’s life cycle involves different people with distinct needs—from clinicians and patients to service technicians and regulatory bodies.
-

Ariana Wilson and Mark Drlik explore how teams can reduce the device development timeline without compromising quality or compliance.
-

Ariana Wilson and Mark Drlik take inspiration from a scene in The Empire Strikes Back to talk about real-world parallels to the Star Wars bacta tank.
-

In this MedDevice by Design episode, Ariana Wilson and Mark Drlik take us back in time to explore iron lung innovation during the polio epidemic of the 1920s.
-

Ariana and Mark examine the complexities of endoscope reprocessing, one of the most difficult tasks in medical device hygiene.