
Interesting 2025 Medtech Trends
Predicting the trends of a new year is always interesting and a bit unpredictable. We asked our medical device design and development professionals to submit their most interesting medtech trends for 2025 and the reasoning behind their prediction. The results were surprisingly focused on two major trends: Home Healthcare and Wearable Devices. Within these categories, several technologies were identified including edge computing, IoT, and connected devices. In no particular ranking, here are our 2025 predictions:
Connected Devices, Edge Computing and Home Healthcare
Connected devices and edge computing are a couple MedTech trends to watch in 2025. These empower small home-use devices to provide higher quality care for patients. This allows patients to recuperate in a comfortable environment while clinicians monitor their status in real time.
At-home healthcare. Baby boomers, one of the largest demographics in history, are healthier than any generation before them. As they reach advanced years, they also have a strong desire to stay home and care for themselves. Because health risks are still present, many companies are moving into at-home monitoring and alerts for healthcare providers. Many more indications could be monitored and treated remotely via at-home visits with new portable and discrete devices. This adds up to a strong business case, but new use cases require novel development utilizing the latest technologies.
IoT, edge computing (a distributed computing model that moves data storage and computation closer to the source of data), and their integration with cloud computing resources can significantly impact the medical device industry. Typically, devices are made as small as possible, but the smaller a device is the less computing power it has. There will be a trend use edge computing to leverage more powerful computational systems in data centers or on-premises computers (vs on the device itself) to process health data and extract valuable insights in real time.
Health Monitoring and Wearables
Personalized health monitoring using wearables and implantable biosensors that enable patients to get more data about their life will be more supportive of Medicine 3.0 (a proposed paradigm shift in healthcare that emphasizes a proactive, personalized, and data-driven approach to health optimization) preventative health care rather than symptomatic treatment. I am a huge fan of getting more information about my own body, my own health. I get very encouraged seeing developments in wearables and sensors like glucose monitors that make patients more educated about their health.
Some of the apps educate patients on how to interpret the data provided by sensors and wearables. For example, they give a scale so the user can determine where they are on the scale of healthy and unhealthy. The apps can also include educational content with actions to improve the patient results.
For very good reasons, there is a gulf between consumer health care apps and medical grade health care apps that a doctor could consider actionable due to regulations. But we’re already seeing signs of medical device company interest in bridging or augmenting that gap to make wearable device data actionable by doctors as well as consumers. This requires applying the same care and oversight as data taken in a clinical setting available in a consumer wearable or an implantable that’s continually logging data.
Once smartwatches and other consumer devices get a pulse oximeter with regulatory clearance, there’s going to be a wave of interest from healthcare practitioners. Because healthcare is becoming a lot more remote thanks to learnings from the pandemic, patients do not see doctors in person as often. If there’s trust in data from smart rings and watches that collect useful information which helps a clinician support patient health needs, especially in a proactive way, let’s have more of that, please!
The gulf between consumer health care apps and medical grade health care apps is going to diminish sooner than later. In the past there wasn’t a lot of connection between consumer grade health wellness devices and what your doctor can see or care about. But that chasm is narrowing.
Wearables and remote monitoring trends are interesting because they let doctors keep an eye on patients in real time from their home. This will help give patients better insights into what’s really going on with their health and makes it easier for doctors to catch potential issues earlier. I think this trend will cut down on hospital visits and make healthcare more efficient.
Impact of New U.S. Leadership
A major medtech trend in 2025 and for the next four years will be a focus on geography constrained resourcing, i.e. less dependency on international supply chain and more focus on regional device requirements. A protectionist climate in the US and the changing, international commerce landscape will likely have a big impact on the world’s largest healthcare market.
Closing Thoughts
One fun and useful thing about being in the medical device design and development business for 25 years is not just getting smarter and better at our craft, it’s looking back at previous medtech trend predictions. Check out these blogs to see how accurate we were in Bold Predictions for Medtech in 2021, Unpacking the Potential of 6 Medtech Trends (2023), and even MD+DI: Medtech Trends to Watch in 2020. We’d enjoy hearing 2025 medtech trends and comments on our predictions from readers.
Astero StarFish is the attributed author of StarFish Medical team blogs. We value teamwork and collaborate on all of our medical device development projects.
Images: StarFish Medical
Learn how StarFish Medical led a consortium that created a Ventilator 2.0 therapy device in record time.
Related Resources

Nick and Joris explore the wide world of ablation technologies—unpacking how each approach works and what it’s best suited for.
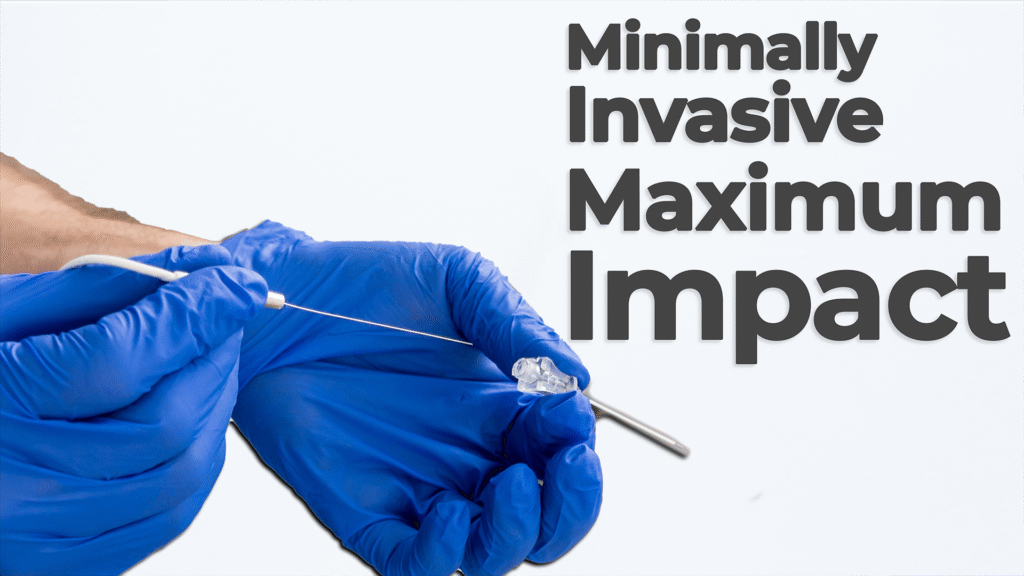
Nick Allan and Joris van der Heijden dive into one of the most impactful trends in modern medtech: minimally invasive surgery. Ablation technology plays a crucial role as hospitals and healthcare providers aim to reduce patient recovery times and overall system strain.
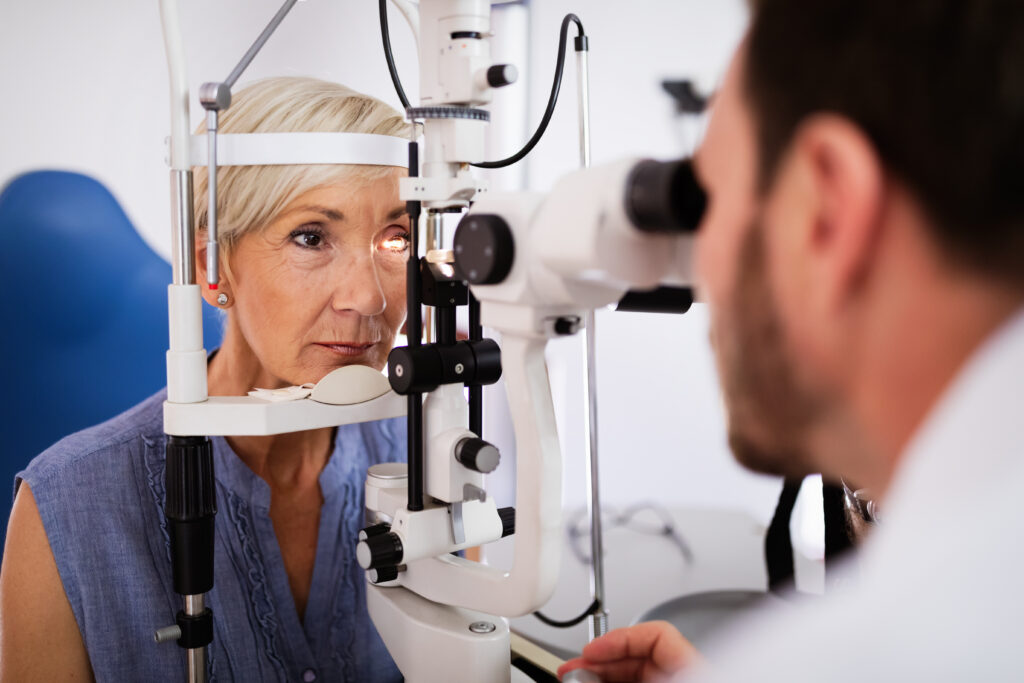
Optics Physicist and Engineer share approaches to performing pre-screen Ophthalmic Instrument Safety Assessment testing in-house.

If you’ve ever been to the hospital, you’ll know that one of the first things hospital staff do is attach “that finger clip device” to your finger. “That device” is called a Pulse Oximeter, and it provides information on pulse rate and blood oxygenation.
Learn the Basics of Wearable Medical Device Adhesives including DO’s and Don’ts in our comprehensive overview.