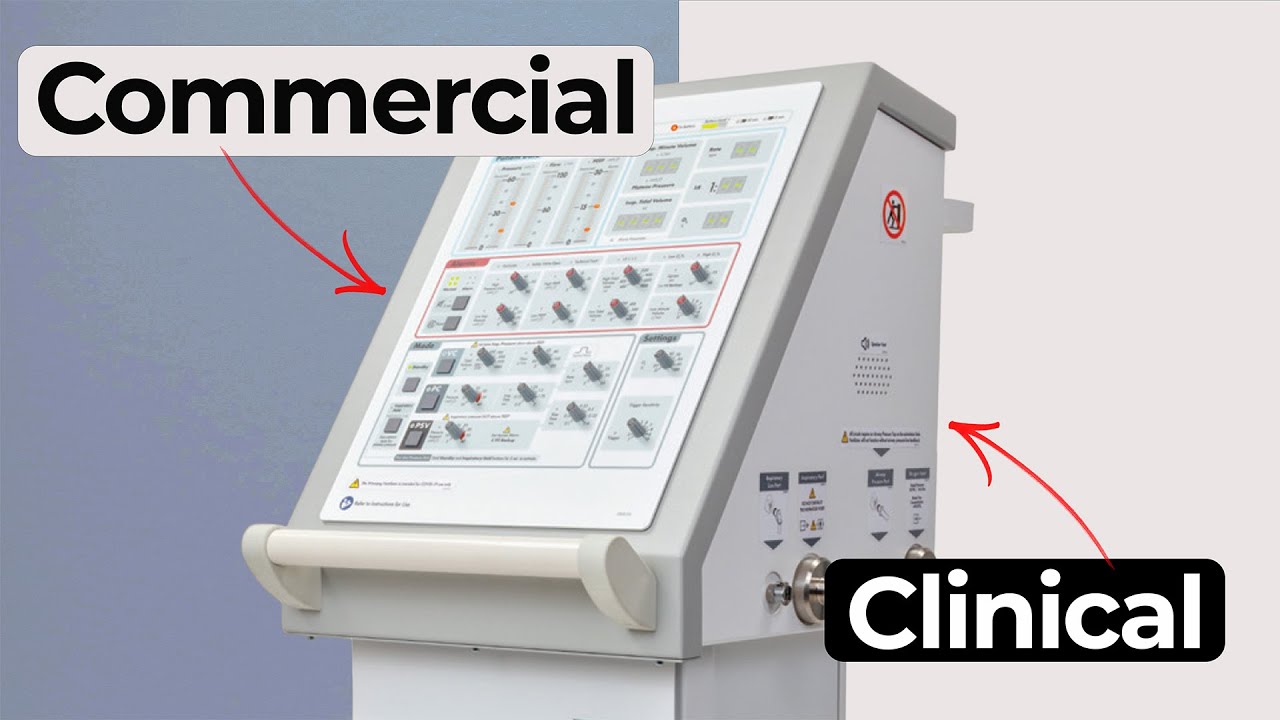
Clinical Ventilator Development Explained
Understanding how clinical ventilator development differs from commercial ventilator design is essential for teams planning early studies. In this MedDevice by Design episode, Ariana and Mark break down the engineering, safety, usability and reliability needs that shape a clinical prototype. They also compare these decisions with the expectations for a commercial device. The discussion provides practical insight into how the clinical ventilator development process supports targeted data collection while remaining safe and effective.
A clinical study device often has a narrow intended use. The team may be evaluating a single feature that supports lung recruitment or another novel capability. This narrow frame influences the user population and environment of use. It also limits which features must be active in the device. Commercial ventilators, in contrast, need a broad feature set that supports a wide range of modes and clinical settings. Ariana notes that commercial systems must remain competitive in the market, which means complex flow control, weaning support and additional modes.
Safety expectations differ as well. A clinical device needs safety systems only for the functionality under evaluation. Biocompatibility testing may not be required for surfaces that will not contact patients during the study. A commercial ventilator, however, must meet full regulatory and regional requirements, including tests for electrical safety and standards such as 60601-1-3 or 60601-1-4, depending on the intended market.
Human factors work also changes. Clinical studies may rely on formative evaluations. Commercial devices usually require a full summative that includes various user groups and languages. Since summatives are expensive for high risk devices like ventilators, it makes sense to limit them during early clinical exploration.
Reliability strategies vary too. Clinical prototypes can rely on on-site support from the development team to address failures. Commercial devices need formal reliability testing, shelf life work for consumables and a defined approach to component durability.
Ariana and Mark close by comparing design for manufacturing activities. Commercial systems need cost optimization and volume assembly planning. Clinical devices do not.
Enjoying MedDevice by Design? Sign up to get new episodes sent to your inbox.
Related Resources

The FDA agentic AI is making headlines after the agency announced its own internal AI review tool. In this episode of MedDevice by Design, Ariana and Mark discuss what this could mean for medical device submissions and regulatory efficiency.
In this episode of MedDevice by Design, Ariana Wilson and Mark Drlik explore what sits beneath that progress, focusing on how these therapies are delivered and why delivery remains one of the hardest problems to solve.

Ariana Wilson and Mark Drlik break down medical vs wellness devices and explain why two products with identical hardware can fall into completely different regulatory categories.

Ariana Wilson sits down with Mark Drlik to unpack why reprocessing is often one of the hardest challenges engineers face during development.