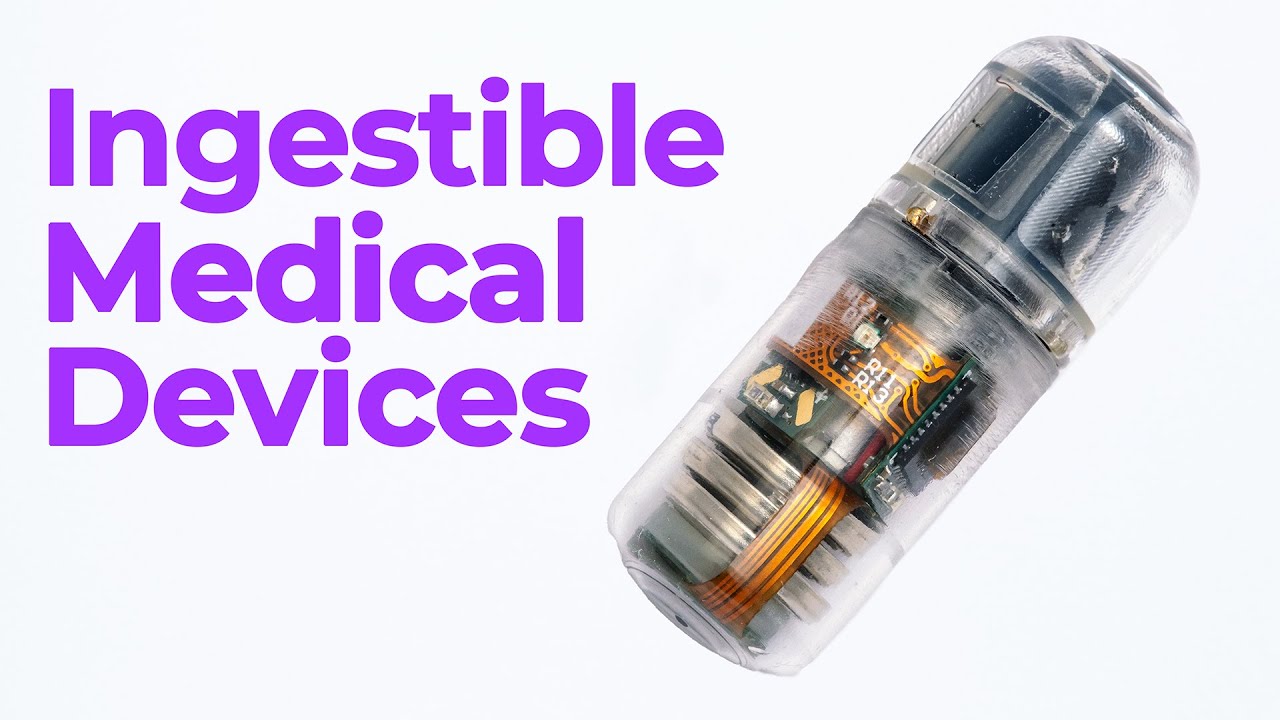
Ingestible Capsules: Future of GI Diagnostics and Drug Delivery
In this episode of MedDevice by Design, Mark Drlik and Ariana Wilson introduce the fascinating world of ingestible capsules—tiny, swallowable medical devices that are revolutionizing gastrointestinal health monitoring and targeted therapy.
What Are Ingestible Capsules?
Ingestible capsules are advanced devices designed to be swallowed and travel through the gastrointestinal tract. Originally developed for imaging purposes, they have evolved to support functions like microbiome sampling, localized drug delivery, and even systemic therapies.
The early pioneers, like Given Imaging, brought capsule endoscopy into clinical use. More than a million people have used these capsules to detect bleeding or abnormalities in the stomach and intestines. Today, innovations allow some capsules to autonomously identify their location using onboard sensors and light reflection, removing the need for external imaging like CT or ultrasound.
Key Innovations and Use Cases
Mark explains how newer capsules can:
- Autonomously identify their position in the GI tract
- Collect biological samples from specific regions
- Deliver drugs to targeted locations
- Break through intestinal walls to deliver systemic treatments
This growing technology is gaining traction for its ability to interact with the complex and previously hard-to-reach environment of the gut.
Development Challenges
Packing motors, batteries, and sensors into a capsule measuring just 11.6 mm by 26 mm is no small feat. Mark compares its computing power to his old Nintendo, showcasing how much performance engineers now fit into a swallowable form factor.
Looking Ahead
The future of ingestible capsules includes more accurate positioning systems, longer battery life, and expanded use in diagnostics, treatment, and monitoring. This episode highlights the ingenuity behind these devices and the opportunities they offer in transforming patient care.
Enjoying MedDevice by Design? Sign up to get new episodes sent to your inbox.
Related Resources

The FDA agentic AI is making headlines after the agency announced its own internal AI review tool. In this episode of MedDevice by Design, Ariana and Mark discuss what this could mean for medical device submissions and regulatory efficiency.

The sandwich ELISA assay is one of the most common ELISA formats used in diagnostics. Nick and Nigel walk through the method step by step using simple visuals and plain language.

For manufacturers of novel devices that can make a significant impact to patient health, the goal of the program is to offer a path to streamlined and potentially faster market entry without sacrificing the rigour around ensuring safety and performance.

When I was starting out in medical devices, the discussion focused on the possibility of an internet of things and the promise of “big data” about everything.