Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
We explore the fascinating intersection of materials science and usability in medical device development. Mark Drlik and Ariana Wilson discuss how anodized titanium produces vibrant color without dyes, and how this visual property supports surgical safety, device differentiation, and biocompatibility.
-
Nick and Joris break down what a DHF is, why it's required, and how it plays a vital role throughout the development lifecycle.
-
Nick Allan and Joris van der Heijden revisit one of StarFish Medical’s most successful Pathfinder journeys, showcasing how a bold research concept evolved into a fully realized clinical diagnostic device.
-
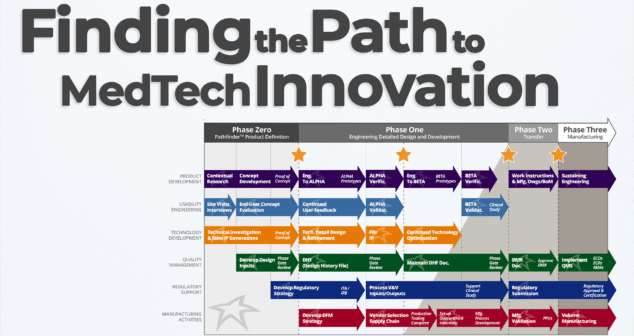
Nick and Joris explore one of the most dynamic early-phase services at StarFish Medical: the Pathfinder Program. If you're a medtech innovator with a promising concept or prototype, Pathfinder helps you identify the right path forward—before you invest millions in development.
-

Nick and Joris explore the wide world of ablation technologies—unpacking how each approach works and what it’s best suited for.
-
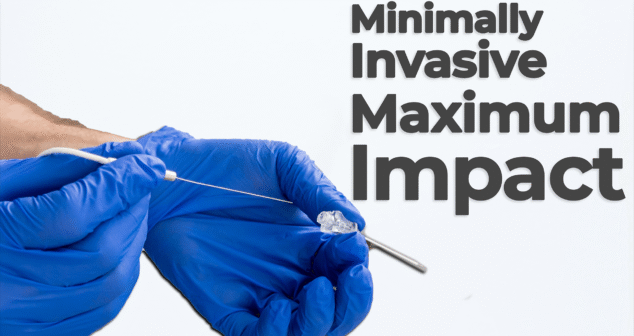
Nick Allan and Joris van der Heijden dive into one of the most impactful trends in modern medtech: minimally invasive surgery. Ablation technology plays a crucial role as hospitals and healthcare providers aim to reduce patient recovery times and overall system strain.
-
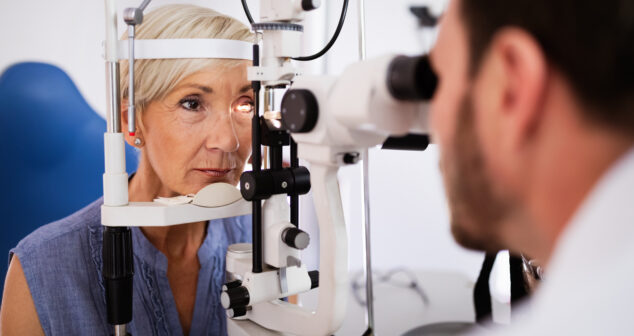
Optics Physicist and Engineer share approaches to performing pre-screen Ophthalmic Instrument Safety Assessment testing in-house.
-

If you’ve ever been to the hospital, you’ll know that one of the first things hospital staff do is attach “that finger clip device” to your finger. “That device” is called a Pulse Oximeter, and it provides information on pulse rate and blood oxygenation.
-
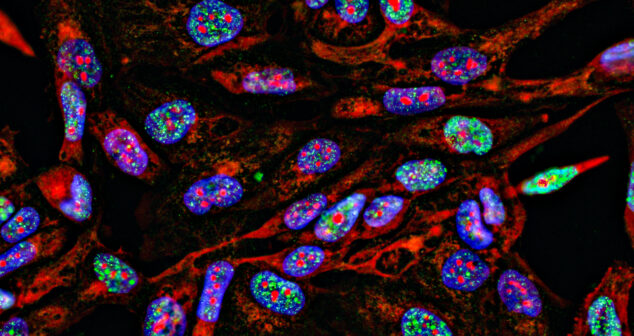
Fluorescence Imaging in Medical Devices outlines medical applications and examples of devices that use fluorescence for imaging.