Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
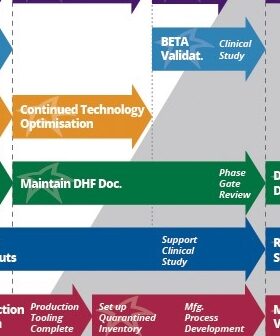
Why build a beta medical device? After a medical device receives regulatory clearance, it’s fairly burdensome to make changes. Beta device benefits are explained by experts.
-
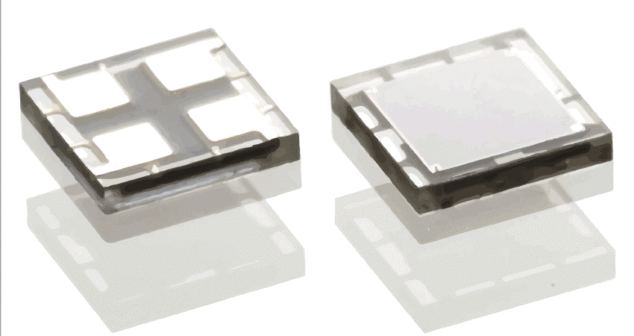
Silicon photomultipliers are an exciting technology enabling applications to be implemented in small, low-cost, low-power form factors.
-
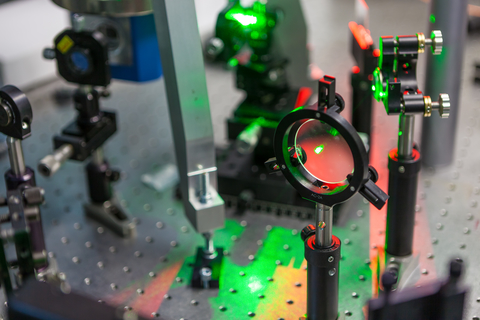
Optics labs best practices to help create an optics lab environment that is clean, organized, efficient, and produces great data.
-

Insights and observations that spark medtech innovation are brainstormed by team of medical device professionals at StarFish Medical.
-

Prototype a medical device on a budget using low-cost tips and methods employed in New Product Introduction (NPI) work.
-

Cellular data connectivity to medical devices? The value proposition is easy to see and the advantages can be well worth the investment.
-

Medical device development experts share their biggest medical commercialization lessons learned over 20 years.
-

Reduce medical device program risks with smart testing strategies – a systematic approach to device testing.
-
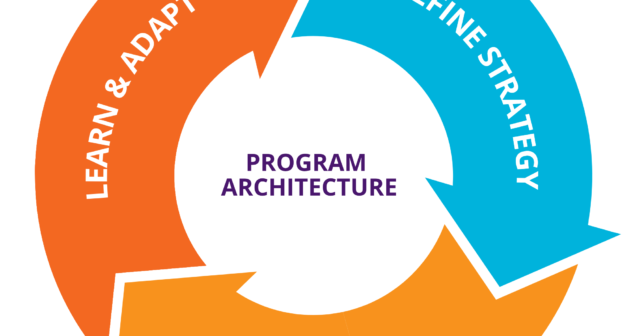
Medical device commercialization projects often need a Project or Program Manager to be the “Program Architect.” of Program Architecture.