Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

In the world of medical device development, unexpected challenges often lead to critical product pivots. In this episode of Bio Break, Nick and Joris discuss one of the most dramatic pivots they've encountered—transforming a lab-developed test (LDT) into a lateral flow assay to expand its market reach.
-

Nick and Joris dive into the fascinating world of freeze-drying, exploring how this process extends shelf life and maintains the integrity of various products—including reagents used in in vitro diagnostics and even instant coffee!
-

What are the most important medical device success factors during development and manufacturing? StarFish employees from QA/RA, NPI, Optics, Computational Analysis, Project Management and Manufacturing answer that question with the factors they deem most important for their area of expertise.
-
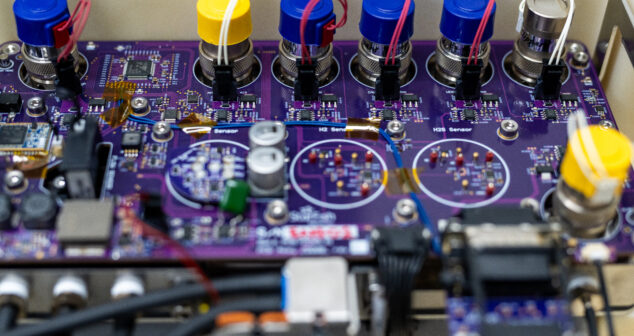
Getting a PCB (Printed Circuit Board) for a medical device right the first time is almost impossible. Datasheets can be misleading, or assumptions and architectures change. As a result, modifications are almost inevitable. Sometimes the modification is as simple as swapping resistors or adding capacitors. Other times it involves tacking on new circuits you had no idea you needed.
-

Medical Device Commercialization Resolutions include Improved communications, aligning goals, using new tools, going paperless
-

Medical device design transfer is a critical phase in the development process, marking the transitory phase from the design and development stage to manufacturing. This phase ensures that the medical device continues to meet its safety, effectiveness, and regulatory compliance once production begins. It is the final phase in the development process and sometimes overlooks or underestimates the amount of time and effort required to ensure that final manufactured devices meet requirements.
-

2024 Top Medical Device Blogs written in 2024 and the 10 most read evergreen blogs during 2024 include three new authors, two group blogs and five articles from blogging veterans.
-

2024 Top Medical Device Videos features a round up of most viewed medical device videos from StarFish Medical includes a mix of new videos released in 2024 along with evergreen videos sharing medical device expertise.
-

In the fast-paced world of medical device development, ensuring a smooth design transfer is critical for product success. However, a common pitfall arises when limits around essential performance are not well defined. In his recent article for Medical Product Outsourcing (MPO), Dana Trousil, StarFish Medical’s Mechanical Engineering Team Manager, dives into the challenges and solutions for addressing these issues.