Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
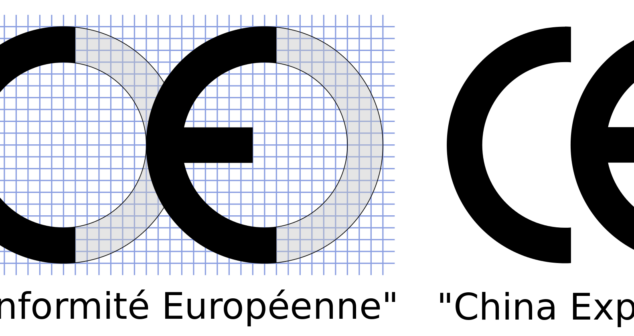
CE vs China Export Mark Explained – Discover meanings, regulations and visual layout of the two marks to avoid costly mistakes.
-
Advice to the novice PCB designer for developing and designing medical device PCB prototypes. Advanced designer feedback is welcomed!
-
Compare 3D printing prototyping merits for three common processes with handy chart and process descriptions.
-

Medical device miniaturization approaches, options and trade-offs when miniaturizing a device’s electronics.
-
The easiest way to decipher comfort when designing a medical device is a combination of irritate testing and asking exact questions.
-

Most clicked StarFish newsletter items during 2016. Now’s your chance to see what everyone else was checking out.
-

When developing a medical device, off the shelf components (OTS) are a good option and avoid reinventing the wheel.
-

What’s the difference between production vs prototype tooling? Consider “What quality do you really need?”, and “How many are you making?”
-
Design engineer’s perspective of medical device biocompatibility to help determine if a device needs to be biocompatible.