Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

A Project Manager navigating this complex medical device development landscape requires the ability to marry traditional Project Management Skills with the specificity of medical device industry practices that focus on patient outcomes and regulatory requirements. This article includes 5 skills that expert project manager, Shalaka Maind, deems crucial to medical device development success.
-

Can’t get your medical device development work done on time? We crowdsourced this dilemma to our employees, and they shared their biggest productivity drains and solutions. Spoiler alert: there were nine drains and five of them share the same solution.
-

Ensuring on-time medical device manufacturing builds are done in a timely manner requires coordinating across several teams including engineering, manufacturing, and quality. Visual Project Management (VPM) and Manufacturing Production Schedules (MPS) help improve manufacturing performance and meet build delivery deadlines.
-
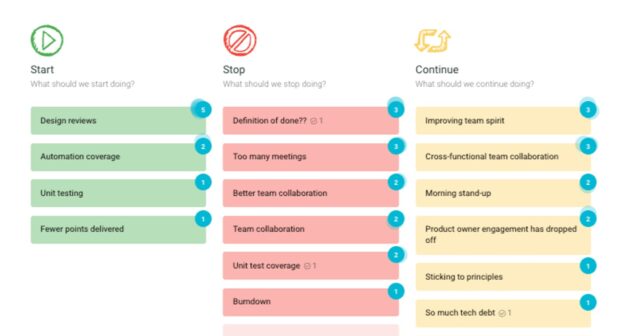
How to ensure team members leave project review meetings feeling seen, heard, understood, and hopeful about future projects. This article discusses four proven project review elements that deliver a game plan to address key issues and drive improvements.
-

Computational modelling and simulation (CM&S) has been used for decades as an assistive tool for medical device design and development. Real-world examples of gathering evidence for submission to regulatory bodies, investigating fluid dynamics, structural, thermal, electro-magnetic, fluid-structure interactions, and other multiphysics problems.
-
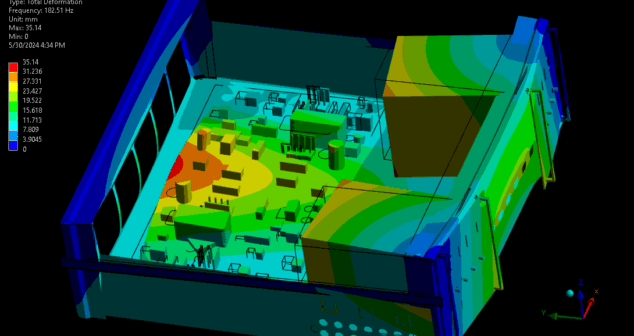
The impact of vibration on medical devices can be understood through a combination of vibration analysis and testing. This article reviews medical devices most susceptible to vibration, provides basic theory and methods of vibration analysis, and summarizes the benefits of using modal analysis when developing medical devices.
-
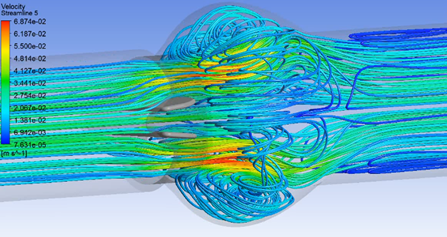
Simulation technologies are revolutionizing the field of heart valve repair and replacement, offering unprecedented insights and capabilities. Enhancing our understanding of valve mechanics, improving device design, optimizing surgical techniques, and enabling personalized treatments, simulations are paving the way for better cardiovascular care and patient outcomes. This article explores the transformative role of heart valve computational simulation in understanding, and treating heart valve diseases.
-

Four areas that can make or break a new medical device development project from the start. Experts identify actions and offer advice that helps ensure new medical device projects start on the right foot.
-

Summary of nine-step process for developing and assessing the credibility of CM&S for regulatory submissions in the FDA Guidance Document “Assessing the Credibility of Computational Modeling and Simulation (CM&S) in Medical Device Submissions (17th November 2023)”.