Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Medical Device Usability Engineering and user engagement during the design and validation process are crucial to success.
-

Proven Process for Medical Device Success– Pathfinder Process: Regulatory, Human Factors and Workflow, Technology Assessment, Design for Manufacture.
-
RoHS 2 and Medical Devices impact design and manufacture. Failure to meet requirements could result in product recalls.
-
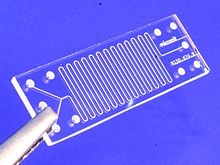
Microfluidic Development Tools in Medical Devices are an example of Medical applications enhanced by a whole host of adjunct technologies.
-

7 Digital Hospital Trends to look for in 2014 and keep abreast of technological advancements for surgical skills and clinical relevance.
-

Reader Favorites: 10 Most Read StarFish Medical Device Blogs of 2013. From FDA Guidelines to EMS pre-screening to Medical Device Margins.
-
Design reviews are a great way to improve the design of a medical device or product. 8 Proven Best Practices are shared.
-

How do we define the quality of a medical device? What attributes cause us to come to the conclusion that a device…
-

Medical device margin matters: In initial medical device product definition always consider the margin in device development discussions.