Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
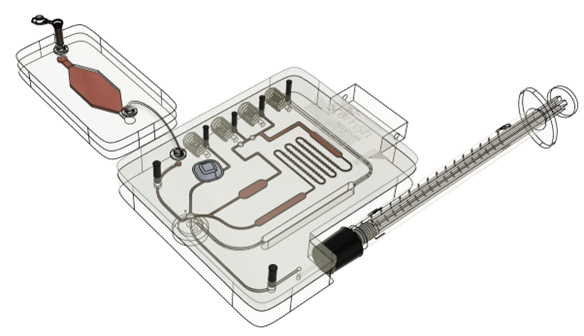
How a ready-made modular proof of concept system for rapid prototyping Point of Care Diagnostics can expedite and de-risk product development.
-

Overview of the Canadian SR&ED program and a 5 step high-level guide on how to prepare an SR&ED tax form (or T661).
-
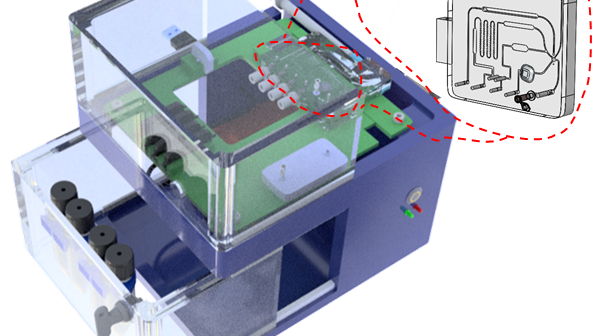
The modular platform for transferring immunoassays acts as an intermediate bridge and is a significant step towards streamlining the product development process for diagnostics.
-
Design and development insights for 3 areas of technology powering the future of cell and gene therapies (CGT) manufacturing: decentralized manufacturing, closed & automated systems and single-use technologies
-
Medical device software is very diverse. In all this variety, one technology is ubiquitous: Git! Three eye-openers which help developers use Git better.
-

Discover eleven essential medical device entrepreneur and founder traits used to successfully navigate the medical device industry.
-

Inventory Risk Management is an important factor for long-term growth and competitiveness in the medical device industry. Learn five key areas to watch.
-

16 important traits and hard and soft skills that medical device experts identify as the most important to medical device development success.
-

The benefits of healthcare gamification and tips to improve patient engagement, access and quality of care.