Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
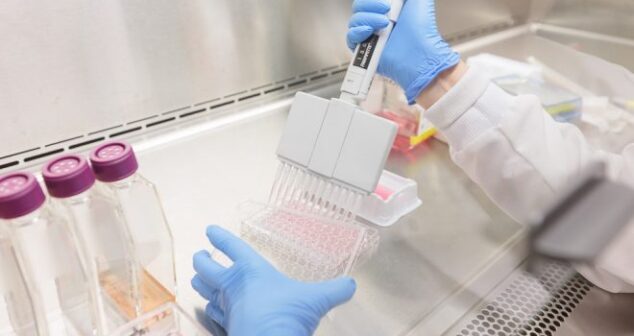
Simulated Fluids for POCT Development enable testing using a liquid that mimics the critical properties of the desired clinical sample.
-
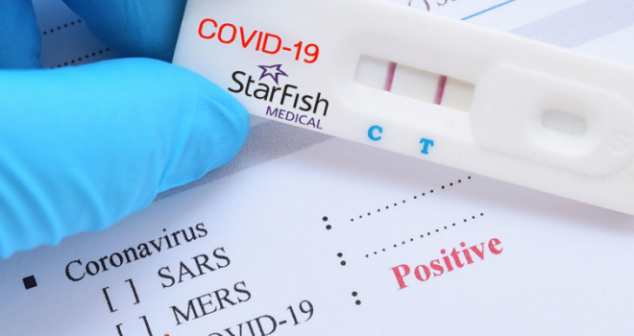
Efficient diagnostic device design requires having a good, well-defined Target Product Profile (TPP) going into the development cycle.
-

Questions and resources to help you find the right medical device development partner for your unique needs and requirement.
-
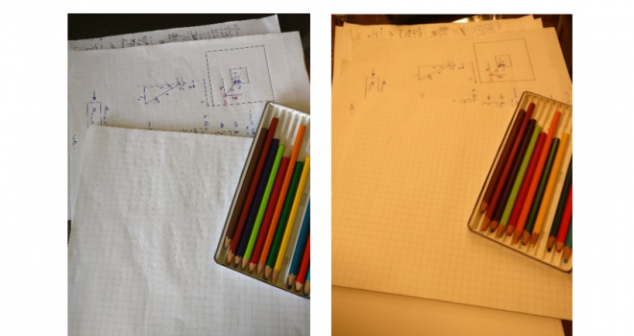
Medical device color management overview of why colour-rendering accuracy is critical for LEDs CRIs and Full-Colour Imaging.
-

Working at StarFish – Guess the top 9 surprises our employees noticed when they first started working at StarFish and still notice after many years.
-

Verify Environmental stress cracking (ESC) quickly, it's one of the most common failure mechanisms in parts made of polymeric materials.
-

RoHS 3 (EU 2015/863) expands the list of prohibited substances from 6 to 10 adding 4 phthalates. Learn if your device is RoHS 3 compliant.
-

Medical Device Case study example where "dig deeper" – IEC 60601 saved the author from potential problems later down the road.
-

FDA ASCA Pilot Program is expected to bring medical devices to patients and users more efficiently by using ASCA-accredited labs.