Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
Medical device artificial intelligence (AI) overview of AI, applications and devices, investment landscape, and regulatory implications.
-
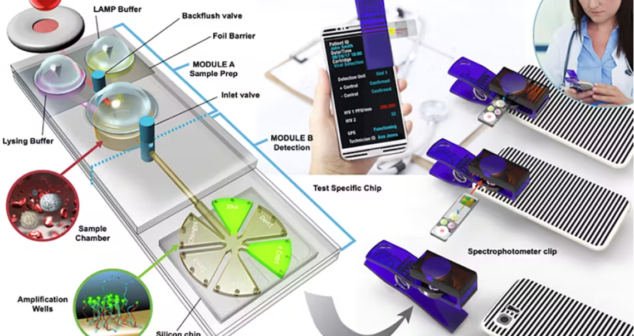
Overview of optical technologies for point of care (POC) medical devices that have been developed or show promise.
-

The path to building a successful medical device startup is rarely straightforward. But sometimes, the most powerful innovations come from personal experiences — moments of challenge, insight, and resilience that drive founders to create solutions that transform lives.
-

Biotech in medical device companies: 3 compelling reasons to pursue a Bio Services career in medical devices.
-

Don't underestimate the value of leadership development
-

Medtech Job hunters during and after COVID should pay attention to these six changes and adjust their search techniques and tools accordingly.
-
An extended recruiting process benefits everybody by finding things that would make a candidate or a company a poor fit for each other.
-
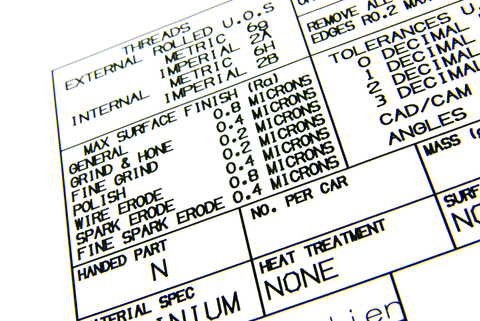
Tolerancing is the consideration of process accuracy (and, to some extent, precision) when designing a device or component.
-
2020 Medical device development videos include COVID implications for research and POC devices, optimizing founder value, manufacturing for NPI, IVD insights.