Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

The costs of early-stage medical device development in North America and Europe continue to rise. Increasing technical complexity and the compounding costs of nonclinical and clinical evaluations are driving this trend.
-

Today, 85% of the top 50 healthcare companies use Computational Modeling and Simulation (CM&S) to develop their products and processes. Whether it’s refining overall device parameters or optimizing critical requirements, engineering simulations help reduce development timelines and enhance design exploration.
-
Computational Modeling and Simulation (CM&S) for medical devices has become a pivotal tool across the medical device industry, complementing and often enhancing traditional bench testing and clinical studies.
-
Nick and Nigel discuss an often-overlooked but increasingly relevant form of drug delivery: suppositories. While typically considered old-fashioned, suppositories are experiencing a resurgence in modern MedTech thanks to their versatility, systemic absorption benefits, and emerging formulation technologies.
-

Mark Drlik and Ariana Wilson introduce the fascinating world of ingestible capsules—tiny, swallowable medical devices that are revolutionizing gastrointestinal health monitoring and targeted therapy.
-

While most people think of Botox as a simple beauty treatment, there’s a surprising amount of engineering, anatomy, and precision behind the process.
-
Ariana Wilson and Mark Drlik dive into how the FDA is adopting artificial intelligence to modernize its regulatory processes. With a new chief AI officer in place and rumors of collaboration with OpenAI, the agency is taking major steps to automate review workflows and improve efficiency.
-
Nick and Joris break down what a DHF is, why it's required, and how it plays a vital role throughout the development lifecycle.
-
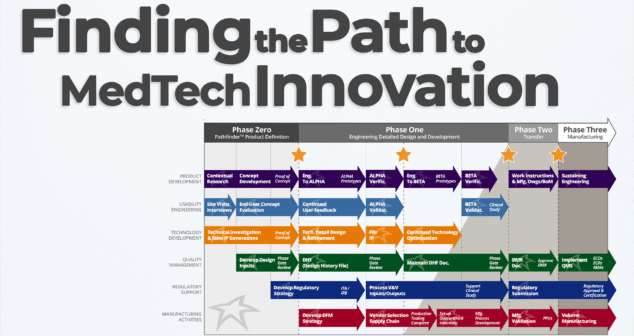
Nick and Joris explore one of the most dynamic early-phase services at StarFish Medical: the Pathfinder Program. If you're a medtech innovator with a promising concept or prototype, Pathfinder helps you identify the right path forward—before you invest millions in development.