Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

11 medical device development mistakes that surfaced frequently in a variety of products. Avoid them to reduce cost and time to market.
-
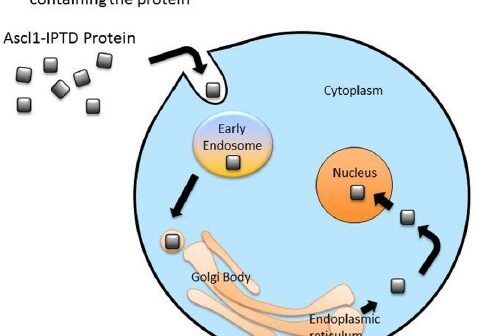
Cellular reprogramming is a strategy for a variety of medical applications where an adult cell is flipped from one type directly into another.
-

When selecting a method for developing an assay, whether it is for detecting bacteria or simulating fluid, the approach all depends on…
-

How to maximize your biotech R&D budget? Form an academic-industrial partnership. 5 tips for a successful academic-industrial partnership.
-
Overview of Simulated Fluids (SF) includes 5 of the most common simulated biological fluid recipes to aid in medical device development.
-
Assay industry trends overview includes infectious diseases diagnostics industry continuing to expand into traditional diagnostic areas.
-
GDPR is designed to enhance the privacy and security of data subjects. Medical devices and GDPR cross paths in many areas.
-

Our employees' medtech resolutions include great ideas. What are your improvement plans? We'd love to hear and share them.
-
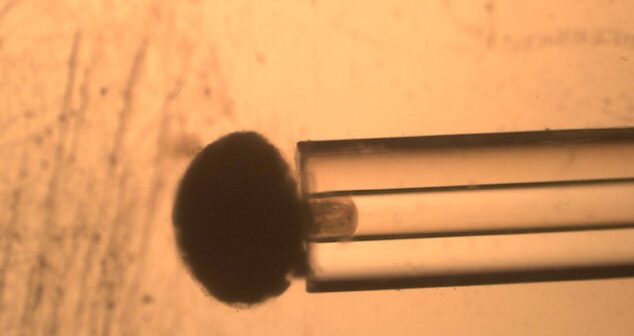
The technique of Micropipette Aspiration is an inexpensive way to measure the viscoelastic properties of cells.