Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

3 Medical UX design differences between the graphical user interfaces (GUI) of digital displays in medical devices and consumer products.
-

How to handle recent medical product development regulatory changes under the 21st Century Cures Act and device exemption list.
-

Start early to avoid surprises when preparing for MDR 2017/745. The new EU medical device regulations will come into full force in Q2 2020.
-

Tips to manage competence and training, keep up to date and ensure personnel are trained and competent to perform their duties.
-

MDSAP Medical Device Single Audit Program audits will help ensure compliance with QMS and reduce the audit/inspection burden.
-
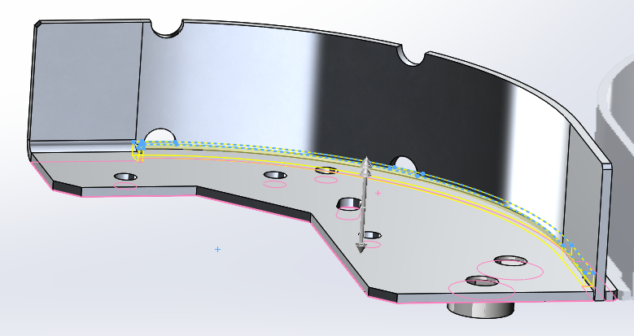
How to prototype medical device parts in 4 steps by using SLS printing to replace traditional sheet metal prototype parts.
-

Comparison of the biggest unknown between IEC 62366:2007 and IEC 62366 -1:2015– Fig. 5.10 User Interface of Unknown Provenance (UOUP).
-

Simulated Fluids help avoid high costs of animal trials and exposure risks to infectious biological for early stage medical device design.
-
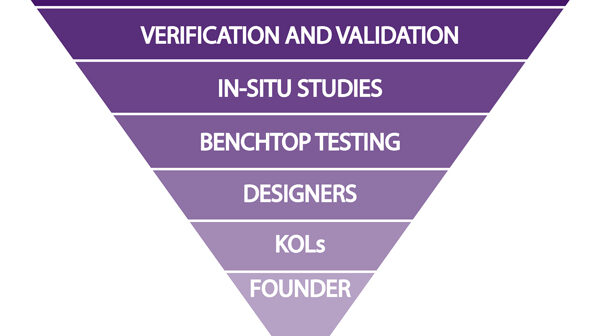
Balancing cost and feedback in medical device development is about when, from whom, and how much weight to give clinical input.