Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

4 tips to collect meaningful user data for emerging medical device technology and take an appropriate level of risk and reward.
-
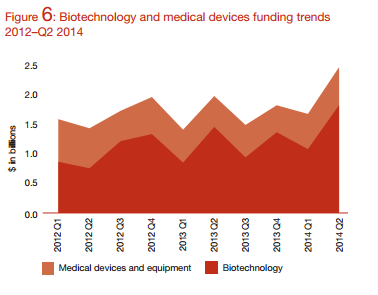
Medical device funding trends for 2014. Analyzes how Medical Device industry is faring for new venture capital investment using PwC MoneyTree data.
-

Project Manager analysis of medical device development client-vendor perception gaps on cost, timeline, quality, and communication.
-

Medical Device injection molding partnering tips and considerations: Validation, Cost Benefit Analysis, Vendor Quality Certification.
-

Selecting the right injection molding partner is crucial. Costs and timescales can be a significant part of a project budget. PT 1 of 2
-

IEC 60601 impact testing requirements are nerve-wracking tests. 6 tips plus video to help understand the methods and avoid any mistakes.
-

Medical Device Development tools to select the right concept and make more effective technical decisions using the Concept Downselect Matrix.
-

Analysis of IEC60601-1-2 (2014) 4th Edition update. Includes examples and impact on medical device regulatory testing and commercialization.
-
Why switch to a medical device specialist manufacturer? Hear from clients who moved to StarFish Medical from a non-medical contractor.