Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

In the highly regulated world of medical device development, ensuring product safety, quality, and compliance is essential. One critical yet often overlooked aspect of this process is test method validation (TMV) in medical device development.
-

A structured, well-documented design review process is a critical component of successful product development, particularly in the medical device industry.
-

In medical device development, we deal with complex projects that span multiple disciplines, timelines, and regulatory gates. It’s a constant balance between moving fast enough to innovate, but slow enough to stay compliant.
-

Ariana and Mark walk through FDA-approved options and explain how to select the right one for your product. From metals to plastics and electronics, not all devices can handle the same process.
-
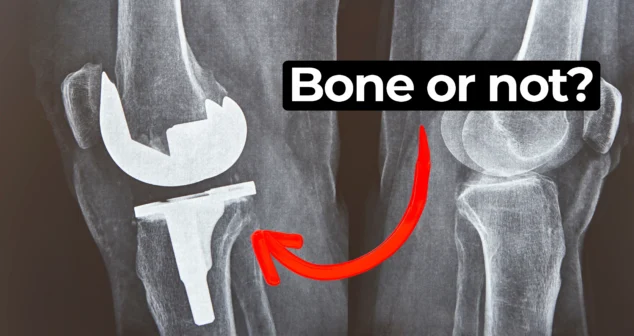
In this episode of MedDevice by Design, Ariana and Mark dive into the biomechanics and materials science behind osseointegration for implants.
-
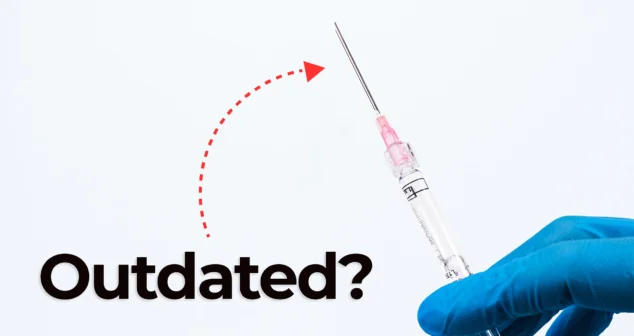
Nick and Nigel dive into the world of jet injector drug delivery. This needle-free method, made popular in science fiction and real-world vaccines, is still used today.
-

Ariana and Mark explore how accommodative intraocular lens technology may one day restore natural vision for people who require cataract surgery or suffer from presbyopia. As Mark shares, traditional bifocals are not ideal, and new lens solutions may offer better outcomes.
-
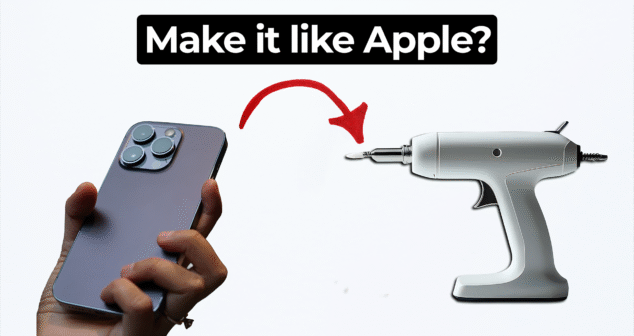
Many clients now request their devices to look and feel like Apple products. But achieving that level of simplicity and elegance is not as easy as it seems.
-
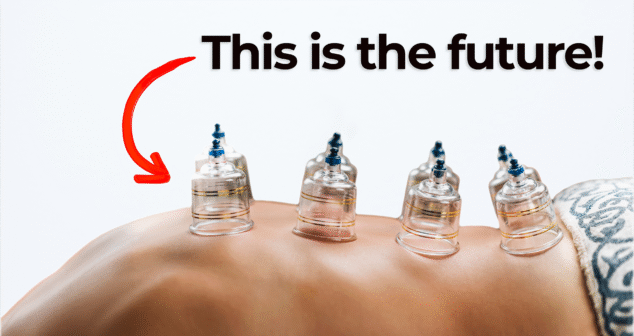
Nick and Nigel explore a surprising approach to injection pain reduction using suction technology. What started as an unusual product order at StarFish Medical led to important insights on improving patient comfort during injections.