Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Zombie Microfluidic Cartridge early indicators to determine if a cartridge will function as designed or fail during the assay protocol run.
-
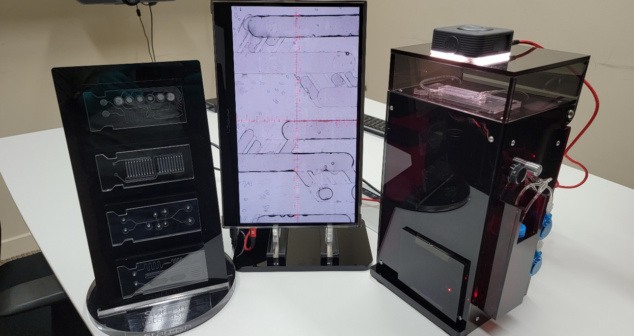
Building an affordable customized Microfluidics Microscope set-up to view droplets as they are being generated.
-

Overview of current and emerging microfluidic technologies as an alternative to traditional methods of cell detection in fluids.
-

Seven Medical Device Commercialization Polarities with tips on how to manage them throughout the development and commercialization process.
-

Integrating Microfluidics into Biomanufacturing overview and top 4 enabling features of microfluidics for biomanufacturing.
-

Five examples of Microfluidic Machine Learning enabling microfluidic technologies to push into new areas and applications.
-

Microfluidics for molecular and serological diagnostic tests enable shorter turnaround times, high accuracy results, and lower cost per test.
-
Medical device artificial intelligence (AI) overview of AI, applications and devices, investment landscape, and regulatory implications.
-
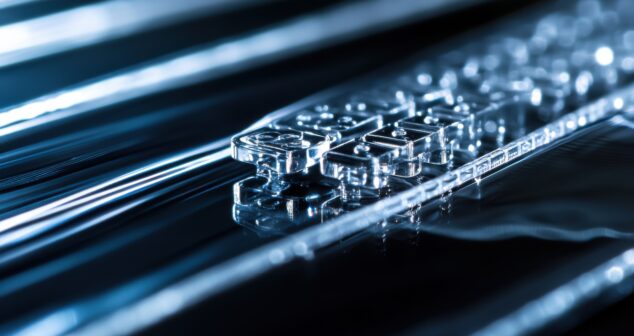
This paper offers insights and case studies in designing and producing a reliable microfluidic device, then taking it through clinical testing to market. It highlights steps for testing device performance (part of regulatory compliance) and setting suitable acceptance criteria for successful market introduction.