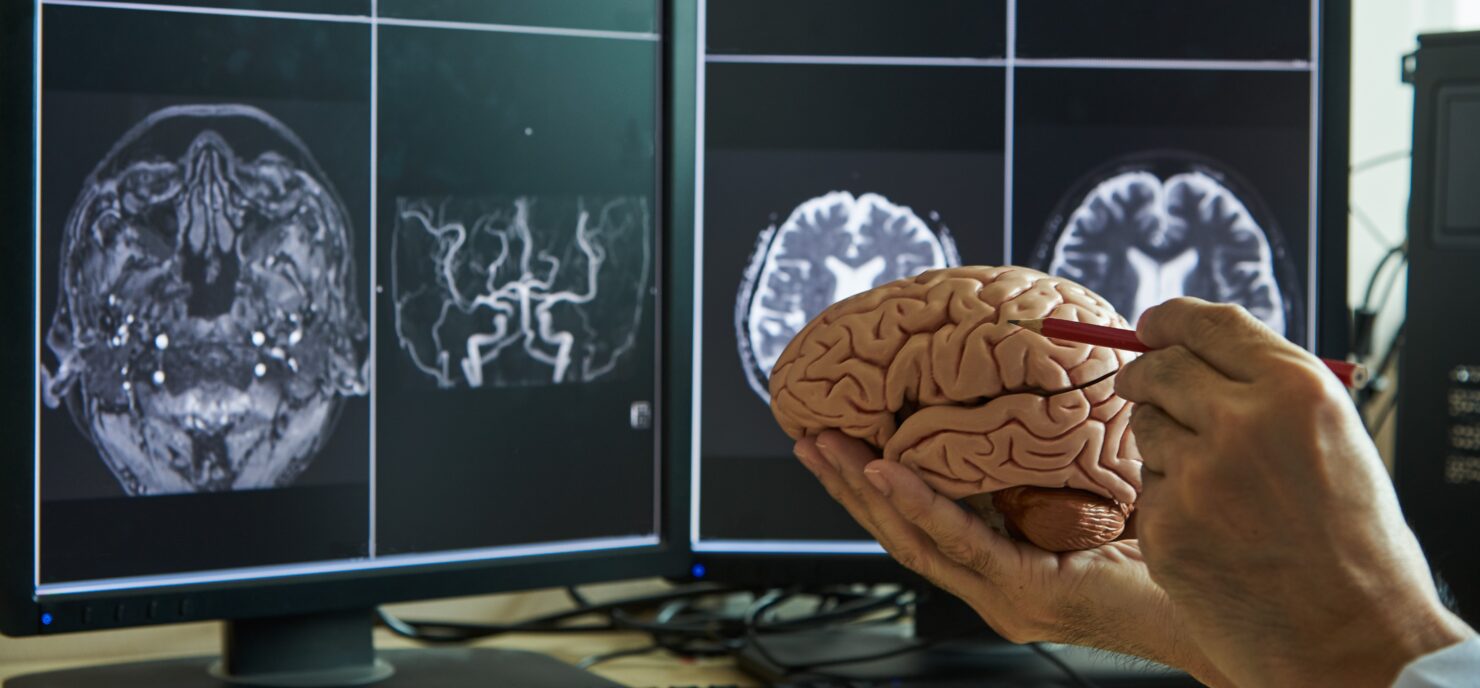
Drug Delivery
Custom solutions for drug delivery devices and combination products.
Your drug is the star. We deliver it with Precision.
In the evolving landscape of cell and gene therapies, biologics, and complex treatment regimens, the method of delivery is as critical as the therapeutic itself.
At StarFish Medical, we specialize in designing innovative drug delivery devices that enhance treatment efficacy, improve patient compliance, and streamline the user experience. Our multidisciplinary team combines technical and scientific expertise with user-centered design to create solutions that bring your therapy to life.

Proven Success in Drug Delivery Devices
StarFish Medical has a track record of successfully bringing drug delivery devices to market, particularly those designed for targeted delivery to specific organs or tumors. Our portfolio includes combination products that enhance usability for at-home users and help reduce patient burden.
Whether your therapy targets a tumor, a neural pathway, or a systemic condition, we know how to translate delivery intent into working devices—built to support the demands of users, trials, and production.
Deep Expertise in Physiology and Pharmacology
Our team includes scientists and market experts with deep knowledge of human physiology, drug stability, and pharmacological dynamics. We don’t just understand the mechanism of action—we understand how your therapy interacts with real-world constraints.
That context informs our design and development process, ensuring devices are engineered with the biology, formulation, and clinical application in mind from the start.
Comprehensive Drug Delivery Services
From early concept definition through regulatory submission and clinical build, we align our work with your drug-device trial programming. Our ISO 13485-certified, FDA-registered facilities support rapid iteration and pilot-scale production in-house, with smooth transitions to your preferred contract manufacturer when needed.
You don’t need to coordinate multiple vendors or reset expectations at every phase. We stay with you through critical milestones and build toward what’s next.
Innovative Outcomes for Our Clients
We partner with startup entrepreneurs and global pharmaceutical companies alike to develop drug delivery devices and systems that overcome technical, physiological, and user challenges.
Examples include:
Explore Our Services
The StarFish Advantage
Trusted partner for drug delivery device development — from early concept to clinical builds and scalable transfer
Our multidisciplinary teams of engineers, scientists, and designers bring the full stack of capabilities needed to solve drug delivery challenges and move confidently from idea to execution.

Custom Solutions for Complex Therapies
Combination products demand tailored thinking. We integrate mechanical, biological, and user considerations into a single, unified development strategy.
Your therapy is unique. So is the solution we build with you.

Aligned with Your Milestones
We adapt deliverables to your clinical and business goals, from IND-enabling studies to investor pitches.
We don’t just meet deadlines—we help define them.

Human Factors & Usability, Built In
From day one, we embed industrial design and usability into your product strategy.
No retrofits. No compromises. Just devices that work for real users.

Agile + Scientific = Executable Innovation
Our agile development process is grounded in scientific rigor and supported by a robust quality system.
You get the creativity of a startup with the discipline of a manufacturer

Manufacturing That Scales With You
We offer rapid prototyping, cleanroom builds, and seamless CMO transfer planning.
Start small, scale smart, and stay in control.

Scientific Insight Drives Better Engineering
Our PhDs and bioengineers collaborate to ensure biological compatibility and device performance are never at odds.
You won’t need to translate between science and engineering—we already do both.
Clinical-Grade Builds Without the Wait
Drug delivery devices often need to be built under cleanroom conditions for preclinical or clinical use. Delays in facility access, inconsistent quality, or handoffs between vendors can jeopardize your trial timeline.
That’s why we maintain ISO-certified, FDA-registered manufacturing facilities in Irvine, Toronto, and Vancouver — enabling faster prototyping, compliant clinical builds, and smoother tech transfer to scale-up partners.
Our infrastructure includes:
- Class 10,000 cleanrooms for clinical-grade assembly
- BSL2 laboratories for safety and performance testing
- Pilot-scale manufacturing to support early trials
- Custom IQ-OQ-PQ protocols for regulatory compliance
- Integrated transfer to large-scale contract manufacturers
Explore Other Sectors We Serve
From lab-on-chip to connected home tests, we help diagnostic innovators bring accurate, accessible technologies to market.
From emergency response to chronic care, we develop therapy devices that improve patient outcomes and meet rigorous regulatory standards.
Ready to explore how we can partner together?
TL;DR
- StarFish Medical is a trusted partner for complex drug delivery device development.
- We specialize in combination products that integrate technical, biological, and usability challenges.
- Our in-house facilities support early-stage development through clinical builds.
- We bring together deep scientific expertise and practical engineering know-how.
- Proven experience across drug classes, delivery routes, and therapeutic areas.
Related Resources


Nick and Nigel walk through how sterile disposables are processed and verified before they reach the field.

The FDA agentic AI is making headlines after the agency announced its own internal AI review tool. In this episode of MedDevice by Design, Ariana and Mark discuss what this could mean for medical device submissions and regulatory efficiency.