
Medical Device Reprocessing Design Challenges
Designing a medical device is never just about performance. Medical device reprocessing design plays a critical role in whether a device can be safely cleaned, reused, and validated in real-world clinical environments. In this episode of MedDevice by Design, Ariana Wilson sits down with Mark Drlik to unpack why reprocessing is often one of the hardest challenges engineers face during development.
Early in the conversation, Mark explains that reprocessing decisions begin at the architecture stage. Teams must decide which parts of a system are consumable and which are reusable. From there, details quickly become complex. Cracks, crevices, adhesives, and sealed interfaces can all make cleaning more difficult. For electromechanical medical devices, designers must also determine whether electronics can be separated or must withstand repeated reprocessing cycles.
Designing for Reprocessing Starts Early
Designing for reprocessing does not wait until the end of development. Instead, it should be considered during early subsystem design and confidence testing. Mark highlights that early testing is especially important when designers already suspect risk areas, such as lenses bonded to surfaces or assemblies joined with adhesives.
However, even verification testing for reprocessing presents challenges. Test soils are often highly controlled and do not perfectly reflect clinical reality. For example, defibrinated blood does not clot, yet it is commonly used in standardized cleaning tests. Achieving realistic mixtures of proteins, carbohydrates, and lipids is difficult, even when following prescribed standards. As a result, verification may pass while still missing real-world variability.
Validation, Observer Effects, and Real-World Use
Design validation adds another layer of complexity. Observable summative evaluations can unintentionally influence results. Mark shares an example where users cleaned devices more thoroughly simply because they were being observed. As a result, competing detergents appeared equally effective, masking real differences.
The same issue applies to medical devices. When users know they are being watched, they often perform cleaning steps more carefully than they would in everyday practice. This creates a gap between validation results and actual use.
Additionally, real-world use does not always follow planned schedules. Emergency procedures, weekend use, and unexpected cleaning intensity can all push devices beyond their validated assumptions. Therefore, reprocessing medical devices requires designers to think carefully about foreseeable misuse and how devices respond to more aggressive or irregular cleaning cycles.
Designing for Misuse and Variability
Ultimately, Mark emphasizes the importance of considering misuse scenarios during design. While it is impossible to test every situation, anticipating variability can reduce risk later. Thoughtful medical device reprocessing design helps ensure devices remain safe, functional, and compliant throughout their lifecycle.
Enjoying MedDevice by Design? Sign up to get new episodes sent to your inbox.
Related Resources

Ariana Wilson sits down with Mark Drlik to unpack why reprocessing is often one of the hardest challenges engineers face during development.
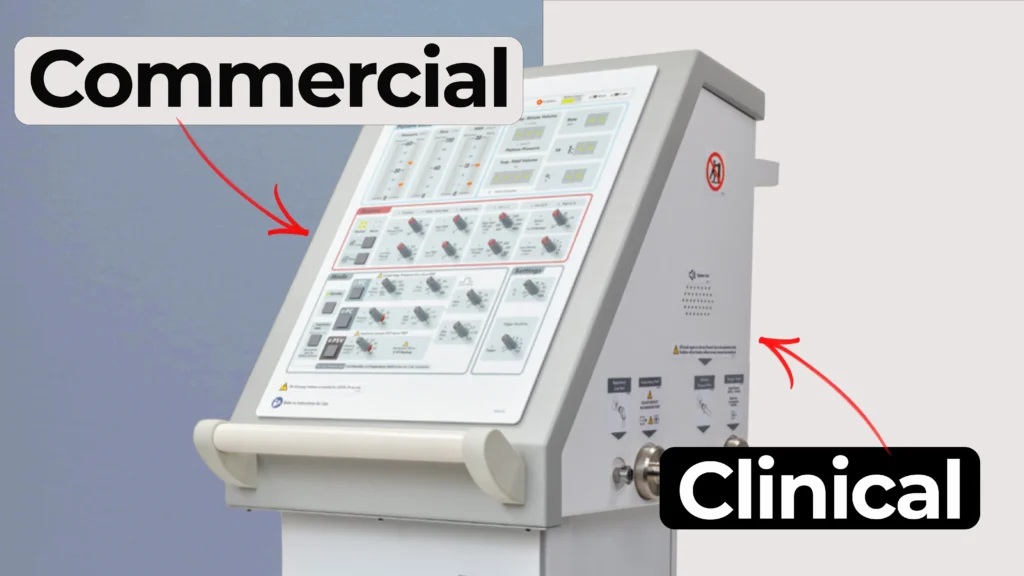
Understanding how clinical ventilator development differs from commercial ventilator design is essential for teams planning early studies.

When Ariana Wilson and Mark Drlik take apart a common appliance, they uncover engineering principles that connect directly to medtech.
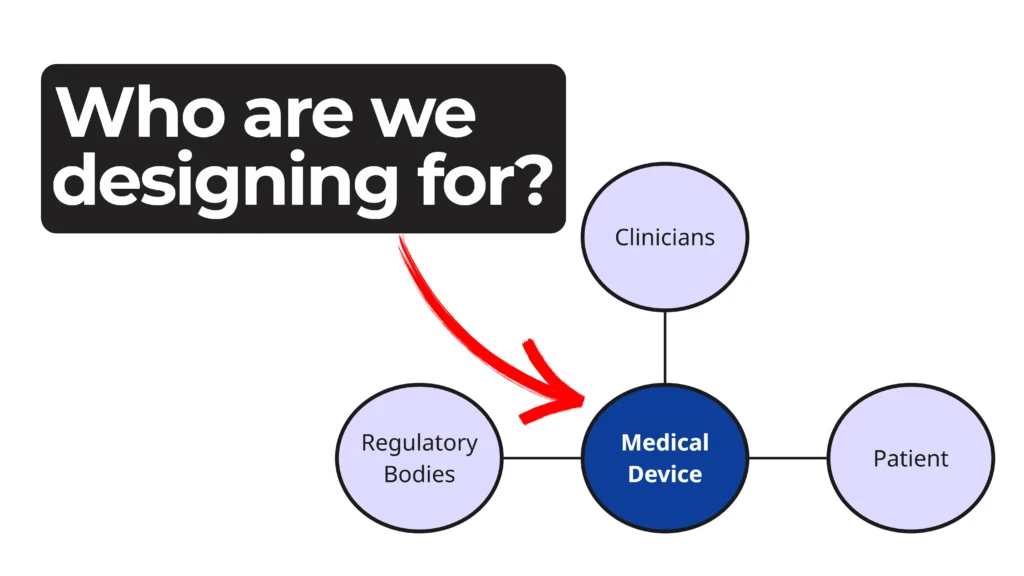
Every phase of a device’s life cycle involves different people with distinct needs—from clinicians and patients to service technicians and regulatory bodies.
