MedDevice by Design
Exploring the Stories Behind MedTech Innovation
Welcome to MedDevice by Design, a video series from StarFish Medical that uncovers the engineering, design, and regulatory choices behind innovative medical devices. Each episode dives into real-world projects and industry trends, bringing you expert perspectives on the challenges and breakthroughs shaping the future of healthcare technology.
Hosted by StarFish Medical’s experienced team, MedDevice by Design is your opportunity to see how medtech ideas move from concept to reality. Whether you’re an engineer, a designer, or simply curious about how devices are made, these episodes deliver practical insights and inspiring stories from the front lines of product development.
Enjoying MedDevice by Design? Sign up to get new episodes sent to your inbox.
Watch our latest MedDevice by Design episodes
Reducing Device Development Timelines
Ariana and Mark explore how teams can reduce the device development timeline without compromising quality or compliance.
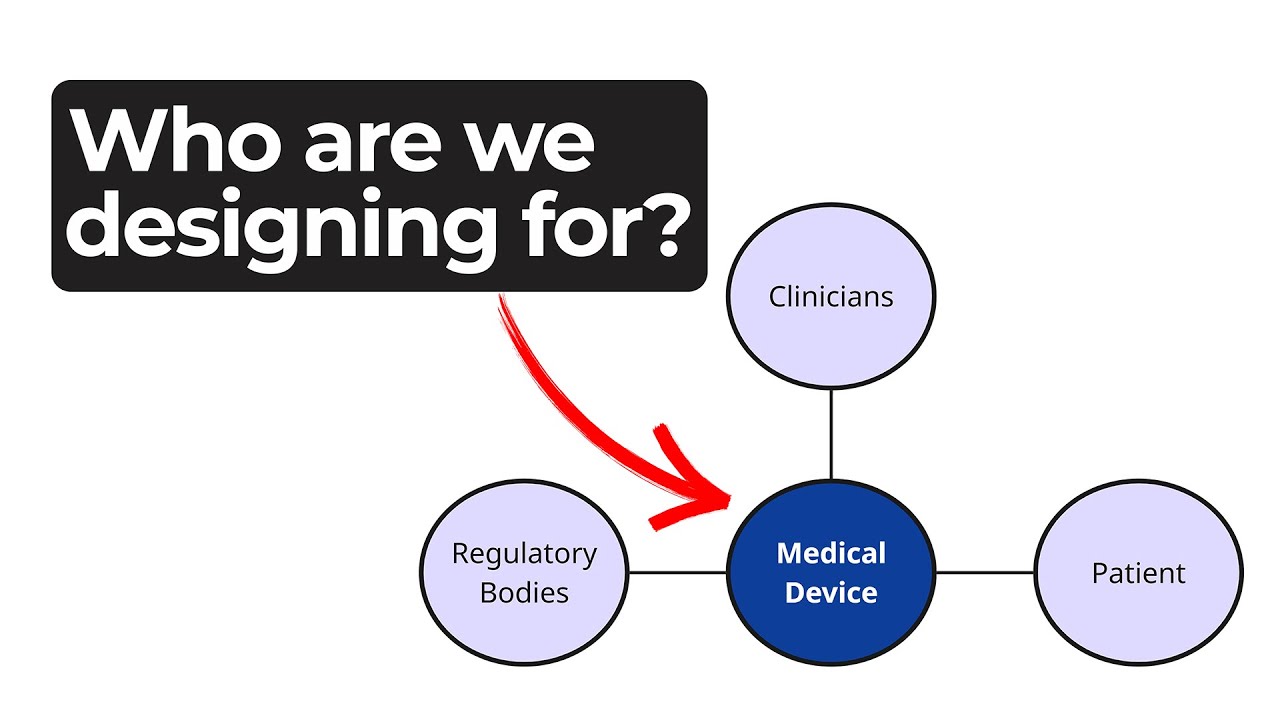
Understanding Medical Device Stakeholders
Every phase of a device’s life cycle involves different people with distinct needs – from clinicians and patients to service technicians and regulatory bodies.
Past Episodes
-

Ariana and Mark explore how accommodative intraocular lens technology may one day restore natural vision for people who require cataract surgery or suffer from presbyopia. As Mark shares, traditional bifocals are not ideal, and new lens solutions may offer better outcomes.
-

We explore the world of brain-computer interfaces (BCIs) and the challenges of capturing thought into action. Mark Drlik and Ariana Wilson walk through how these systems translate brain activity into control signals for devices—without needing surgical implants.
-
Mark and Ariana explore the surprising versatility of barium sulfate—a material used widely in both diagnostic procedures and medical device manufacturing. While many recognize it as the contrast agent you drink before an X-ray, it’s also a key additive that enhances plastic components across the healthcare industry.
-
We explore a groundbreaking shift in how Alzheimer’s disease may soon be diagnosed. Instead of relying on invasive spinal taps or costly PET scans, researchers have developed a blood test that detects key proteins associated with the disease—offering a more accessible and patient-friendly screening method.
-

We explore how breath testing in medical devices is transforming diagnostics. Mark Drlik walks through how this technology supports everything from roadside impairment detection to gastrointestinal analysis.
-

Mark Drlik and Ariana Wilson introduce the fascinating world of ingestible capsules—tiny, swallowable medical devices that are revolutionizing gastrointestinal health monitoring and targeted therapy.
-
Ariana Wilson and Mark Drlik dive into how the FDA is adopting artificial intelligence to modernize its regulatory processes. With a new chief AI officer in place and rumors of collaboration with OpenAI, the agency is taking major steps to automate review workflows and improve efficiency.
-

In this episode of MedDevice by Design, we follow the development journey of a transformative chest therapy device for cystic fibrosis patients. Host Mark Drlik introduces a voice coil prototype from an early-stage project that would eventually evolve into the Hill-Rom Monarch—a commercial system delivering high-frequency chest wall oscillation therapy.
-
We explore the fascinating intersection of materials science and usability in medical device development. Mark Drlik and Ariana Wilson discuss how anodized titanium produces vibrant color without dyes, and how this visual property supports surgical safety, device differentiation, and biocompatibility.