MedDevice by Design
Exploring the Stories Behind MedTech Innovation
Welcome to MedDevice by Design, a video series from StarFish Medical that uncovers the engineering, design, and regulatory choices behind innovative medical devices. Each episode dives into real-world projects and industry trends, bringing you expert perspectives on the challenges and breakthroughs shaping the future of healthcare technology.
Hosted by StarFish Medical’s experienced team, MedDevice by Design is your opportunity to see how medtech ideas move from concept to reality. Whether you’re an engineer, a designer, or simply curious about how devices are made, these episodes deliver practical insights and inspiring stories from the front lines of product development.
Enjoying MedDevice by Design? Sign up to get new episodes sent to your inbox.
Watch our latest MedDevice by Design episodes
Reducing Device Development Timelines
Ariana and Mark explore how teams can reduce the device development timeline without compromising quality or compliance.
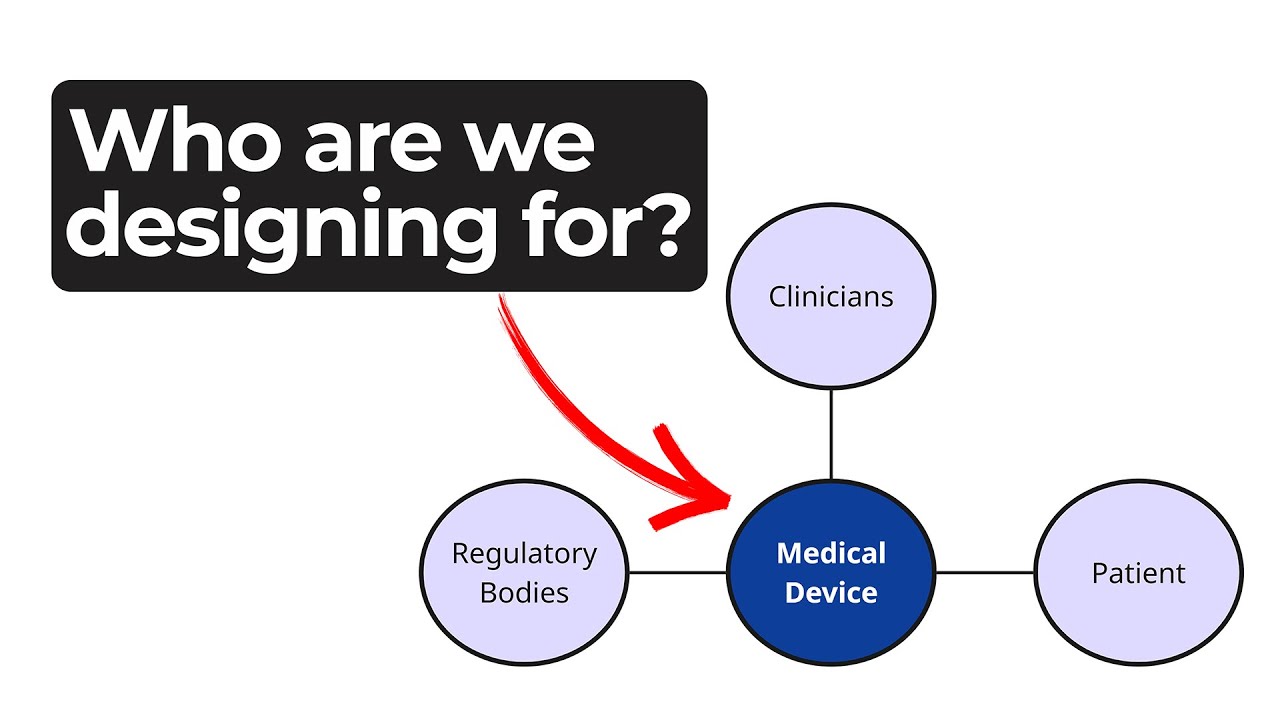
Understanding Medical Device Stakeholders
Every phase of a device’s life cycle involves different people with distinct needs – from clinicians and patients to service technicians and regulatory bodies.
Past Episodes
-

In this episode of MedDevice by Design, Ariana Wilson and Mark Drlik examine what happened, what it means for medical device innovators, and how the FDA’s ASCA (Accreditation Scheme for Conformity Assessment) program helps reduce regulatory risk.
-
Ariana Wilson and Mark Drlik break down a powerful visual framework for understanding what makes a medtech product, and the company behind it, truly successful. Using a triple Venn diagram, Mark explains how strategic alignment across feasibility, viability, and desirability can drive better product outcomes and business success in the medical device industry.
-

What if the next leap in brain therapy didn't require open surgery? We explore how convection-enhanced delivery (CED) is changing the way clinicians administer therapeutic agents to the brain. Join us as we look inside this advanced technique—and the high-precision tools that make it possible.