An electrical engineer’s overview of IEC 60601-1 Terminology & Definitions through Annexes
This blog is part 2 of my Electrical Engineer’s overview of one of the more important standards for medical devices: IEC 60601-1.
We previously discussed the first two sections in Part 1. The Terminology & Definitions section is very useful; any term written in Small Caps is defined in section 3. It is here for example that we find that an Applied Part is a “part of ME Equipment that in Normal Use necessarily comes into physical contact with the Patient for ME Equipment or an ME System to perform its function.”
In Section 4 General Requirements we learn that manufacturers must now employ ISO 14971 Risk Management techniques, which is a significant departure from edition 2 of IEC 60601-1 in which safety was mainly proven through testing. The Essential Performance of the device shall be defined, which is functionality whose loss or degradation results in unacceptable risk. This ties in with the concept of being Single Fault Safe, in which any single failure in the device does not cause an unacceptable risk.
Section 5 General Requirements for Testing requires that testing be conducted under the least-favourable operating conditions. Tests may be omitted if the condition being tested has been adequately evaluated by other tests or methods. The Risk Management File, prepared during product design and prior to testing, plays a large role in documenting the particular conditions and faults that need to be tested.
Section 6 Classification of ME Equipment deals with categorizing the class of equipment (I or II); the type of Applied Part (B, BF or CF); the protection against fluids and dust (Ingress Protection IP rating); and whether or not it requires Sterilization and is suitable for use in Oxygen-enriched atmospheres. The results of this categorization determines the types and limits of the tests that need to be performed – for example, the patient leakage current limits depend on the type of applied part. One typically goes through this exercise of classifying key components early in the design process.
Section 7 on Equipment Identification, Marking and Documents contains requirements on measurement units, symbols used, durability and legibility, and contents of the instructions for use. This IEC 60601-1 section governs, for example, the information provided on the label typically attached to a device near its power cord entry.
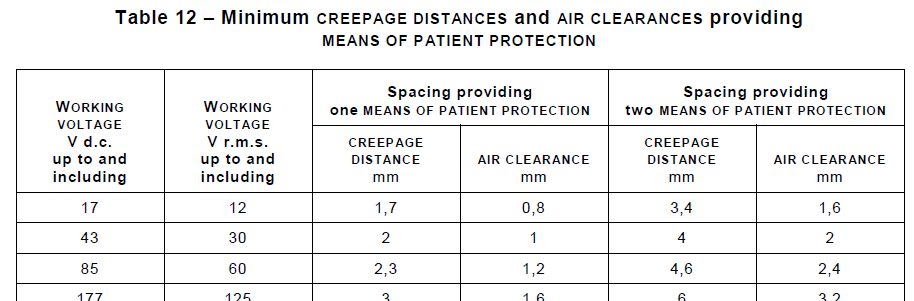
Section 8 Protection Against Electrical Hazards introduces the concepts of Means Of Patient Protection (MOPP) and Means Of Operator Protection (MOOP) and how two means of protection are employed to prevent leakage current and other limits from being exceeded. Tables list items such as the required Creepages and Clearances needed at different Working Voltages, and this section provides many illustrations of test configurations used in verifying compliance. StarFish has adopted many of the IEC 60601-1 diagramming conventions in creating device Isolation Diagrams and other illustrations used in documenting a design – a subsequent blog will likely discuss this in more detail.
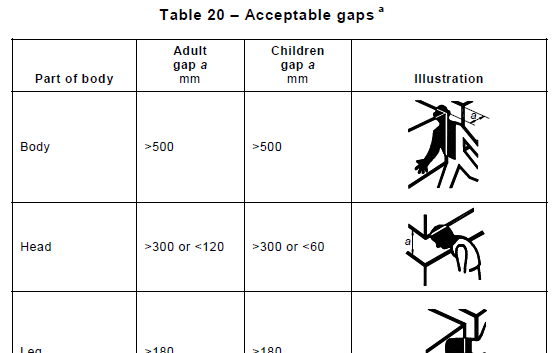
Section 9 Protection Against Mechanical Hazards confirms that safety of ME Equipment doesn’t involve only electrical hazards. In this section are covered requirements relating to Moving Parts, Trapping Zones, Emergency Stops and Stability. Some of the tests depend on the type of device (i.e. Handheld, Portable, Mobile etc) and can be quite exciting to execute – for instance those covering impacts with a steel ball (see Nigel’s blog ), or ones where a cart is rolled at speed over an abrupt drop in the floor.
Section 10 Protection Against Radiation Hazards covers IR, UV, Microwave, X-rays, and Particle Radiation. Because there are many medical devices that intentionally emit radiation, and because measuring radiation can be quite complex, multiple other standards may need to be consulted in conjunction. For example, if lasers or high-power LEDs are employed then IEC 60825-1 or IEC 62471-1 may also be applicable.
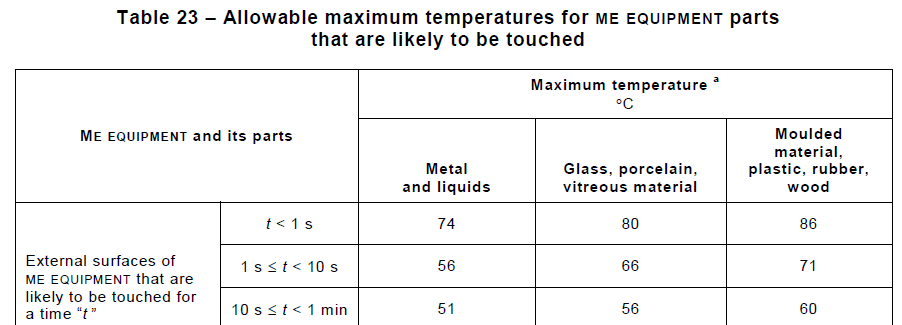
Section 11 Protection Against Excessive Temperatures & Other Hazards has, as one might expect, the requirements for maximum temperatures of parts (which depend on what material the part is made of, and how long the contact duration may be). It also covers Fire Prevention and Flammability, Liquid Overflow, and Biocompatibility (in the latter case, it refers to the ISO 10993 standards).
Section 12 Accuracy of Controls recognizes that Usability Engineering is crucial in the design of a safe medical device. This view echoes that in the FDA guidance ‘Applying Human Factors and Usability Engineering to Medical Devices’. As this is such an important topic, several StarFish blogs have covered this issue (Christine’s and James’ blogs are two of the many).
Section 13 Hazardous Situations and Fault Conditions lists specific hazards, such as emission of flames, molten metal, poisonous or ignitable substances that are not permitted to occur under Single Fault Conditions. Note however that just because a specific hazard is not included in this section does not imply that it is permitted; a complete Risk Analysis should identify such a hazard and create mitigations for it.
Section 14 Programmable Electrical Medical Systems (PEMS) covers the broad topic of safety in devices containing firmware or software. One big challenge is designing suitable tests for assuring safety of these systems, and so this section covers the contributions of the Risk Management Plan, the PEMS Development Life Cycle, and Validation and Verification activities. As well, IEC 62304 (Medical device software – Software life cycle processes) is an essential reference when designing PEMS. Kenneth’s blog discusses some of the difficulties with 3rd party software and how a design process can deal with them.
Section 15 Construction of ME Equipment details the requirements for Serviceability, Impact and Drop Tests, Cords and Connectors, Batteries, and Transformer Construction. For example, foot controls used in operating theatres need to have ingress protection to at least IPX6 per IEC 60529 tests.
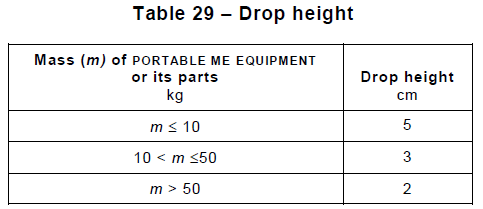
Section 16 ME Systems covers the requirements arising when one connects multiple ME devices with data or power cords, which can affect, for instance, the touch and leakage currents. It also specifies additional documentation, separation devices, and power supply requirements arising from the interconnection.
Section 17 Electromagnetic Compatibility (EMC) is last, but not least. It deals with how performance and safety can be affected by the often-invisible electromagnetic phenomena of electrostatic discharge (ESD), radiated and conducted immunity, and power line perturbations. No specific requirements are listed in IEC 60601-1; instead, it refers to the collateral standard IEC 60601-1-2.
Finally, the Annexes are a rich source of information – in fact, they comprise about 1/2 of the total size of the standard. They fall into Informative and Normative (i.e. specifying requirements) categories. Of particular value is Annex A General Guidance and Rationale, which discusses the reasoning behind many of the IEC 60601-1 requirements. When preparing a Risk Management File for a new design, it often helps to understand the rationale behind particular requirements so that one can design effective mitigations. Other useful annexes show commonly-used symbols for marking controls, give diagrams for measuring various parameters like Patient Auxiliary Current, and discuss aspects of ME Systems.
I hope this overview of the contents of IEC 60601-1 has demonstrated that it is not just an electrical safety standard. At StarFish we use several checklists and templates to ensure we address all of its comprehensive requirements. We are planning some further blogs to discuss these tools. Feel free to contact us with your feedback and any requests for future topics.
Bjarne Hansen, MESc. is a former StarFish Medical Senior Electrical and Biomedical Engineer. An avid sailor, he is also author of our most read blog for over 5 years: Inexpensive prescreening strategies for medical device EMC compliance.
Images: StarFish Medical
Another key requirement for ISO 60601-1 is the protection against ingress. Read more here.