Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

In this episode of MedDevice by Design, Ariana Wilson and Mark Drlik examine what happened, what it means for medical device innovators, and how the FDA’s ASCA (Accreditation Scheme for Conformity Assessment) program helps reduce regulatory risk.
-

In this episode of Bio Break, Nick Allan and Joris van der Heijden explore a critical but often overlooked topic in healthcare innovation: prevention. While most conversations about medical devices revolve around treatment, the duo shifts the focus to technologies that help people avoid hospitalization altogether. Preventive medical devices and diagnostic tools are quietly transforming healthcare by catching diseases earlier and reducing the need for invasive procedures.
-
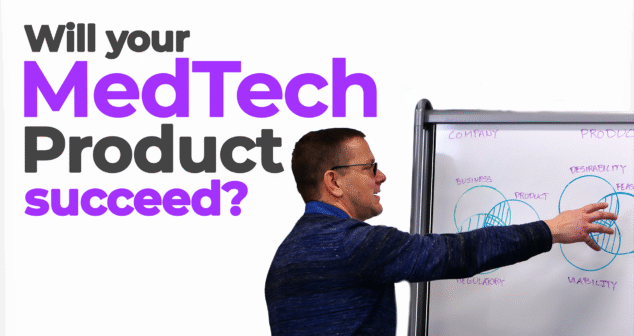
Ariana Wilson and Mark Drlik break down a powerful visual framework for understanding what makes a medtech product, and the company behind it, truly successful. Using a triple Venn diagram, Mark explains how strategic alignment across feasibility, viability, and desirability can drive better product outcomes and business success in the medical device industry.
-

In this episode of Bio Break, Nick shares one of his favorite discoveries in the world of infectious disease research — the groundbreaking discovery of Helicobacter pylori and its role in causing peptic ulcers. This fascinating story showcases how persistence, scientific curiosity, and innovative thinking can lead to discoveries that reshape medical science.
-

What if the next leap in brain therapy didn't require open surgery? We explore how convection-enhanced delivery (CED) is changing the way clinicians administer therapeutic agents to the brain. Join us as we look inside this advanced technique—and the high-precision tools that make it possible.
-

What are the real costs of developing a medical device? In this episode of Bio Break, Nick and Joris dive into one of the most frequently asked questions they hear from clients: How much does it cost to develop a medical device?
-

Nick and Joris tackle a question many tech and health enthusiasts have wondered for years: Where is my cortisol-sensing smartwatch? Nick shares a nostalgic story of reading about futuristic wearable technology in Popular Mechanics as a child — devices that would one day monitor biomarkers like cortisol to track stress and overall health. Now, decades later, he and Joris break down why such a wearable device still hasn't become a reality.
-

We all know medical devices have labels, but how often do we consider their purpose and the effort required to ensure they provide the right information? Device labelling serves as the interface between the manufacturer, the user, and regulatory bodies. (Note that being from Canada, we spell labelling with two Ls.)
-

Sterilization is a critical process in the medical device industry as it provides a reliable way to ensure that devices are free from harmful microorganisms when they are used on patients. This blog talks about the categories of sterilization currently used on medical devices in manufacturing settings. It also addresses concerns surrounding the use of ethylene oxide (EtO), an indispensable method for sterilizing heat and moisture sensitive devices.