Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
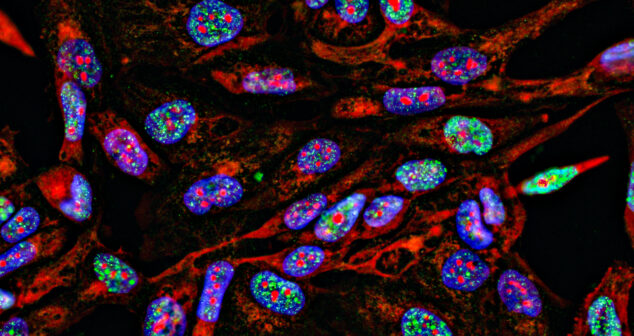
Fluorescence Imaging in Medical Devices outlines medical applications and examples of devices that use fluorescence for imaging.
-
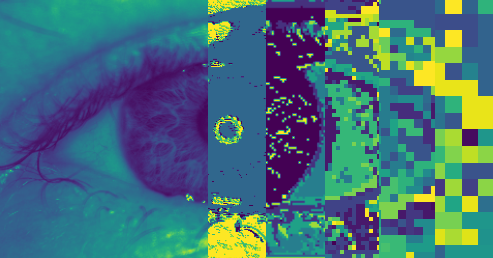
Computer Vision for Medical Devices is constantly evolving and incorporating new techniques and technologies as they emerge.
-
In this episode of Bio Break, Nick and Joris dive into the rapidly growing field of minimally invasive medical technologies, focusing on…
-

Being able to control the release rate of a target molecule is a valuable tool for engineering tissues and therapeutic delivery of regenerative medicine applications.
-
In this episode of Bio Break, Nick and Joris dive into one of the most astonishing—and real—medical innovations we’ve ever come across: osteo-odonto-keratoprosthesis. Or, as Nick quickly dubs it, “tooth in eye surgery.”
-

For Earth Day 2025, we asked our employees to share medtech recycling and innovation opportunities and obstacles. We invite everyone in medical device development and healthcare to unite behind medtech recycling and innovation.
-

The human eye is an extremely delicate organ, often prone to irritation, dryness and various diseases, such as glaucoma, cataracts, keratoconus, age-related macular degeneration, and many others. These ocular clinical conditions also affect patients’ quality of life.
-
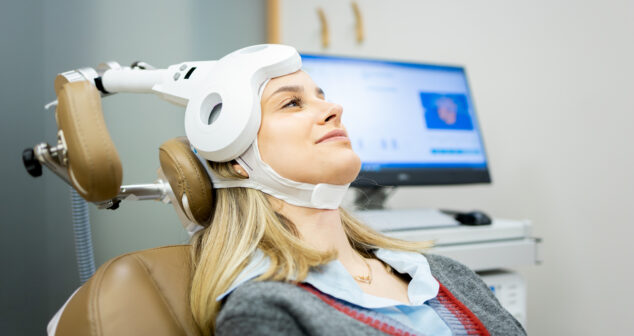
Exploration of drug-device combination therapies that are transforming the treatment of Parkinson’s, epilepsy, depression, and brain cancer.
-
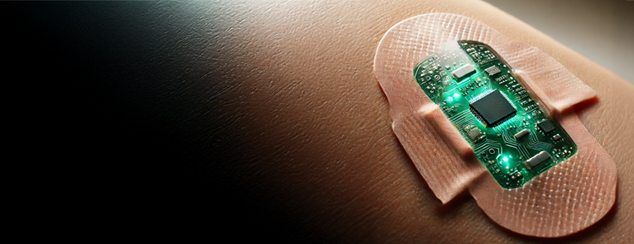
With the recent developments and seemingly ubiquitous nature of real time glucose monitoring and availability of smart wearable tech, the development of a theranostic band-aid seems inevitable. But how practical would this be? Is there a Theranostic wound dressings market?