Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

In this episode of Bio Break, StarFish Medical experts Joris van der Heijden and Nick Allan celebrate Thanksgiving by sharing a heartwarming and hilarious family tradition: preparing the perfect turkey. Through this tale of generations working together to create the iconic holiday meal, they uncover a surprising lesson that applies to medical device development and procedural design.
-

In this episode of Bio Break, Joris van der Heijden and Nick Allan continue their exploration of nature-inspired innovations, focusing on one of the most transformative breakthroughs in molecular biology: the polymerase chain reaction (PCR) and its key component, Taq polymerase.
-

In this episode of Bio Break, StarFish Medical experts Joris and Nick dive into the transformative concept of Continuous Analyte Monitoring (CxM) and its growing role in wearable medical devices. They discuss the value of tracking metabolic markers over time, as opposed to relying on static time-point measurements, and how this approach enhances precision in both diagnostics and treatment.
-

In this episode of Bio Break, StarFish Medical experts Joris and Nick tackle an intriguing question: can alternative bodily fluids like sweat, saliva, or urine offer viable alternatives to blood sampling for medical diagnostics? While blood remains the gold standard for clinical testing, advancements in non-invasive sampling methods are opening new possibilities, particularly in wearable devices and at-home diagnostics.
-

In this episode of Bio Break, Nick and Joris dive into the fascinating realm of biosensors, showcasing how nature’s biological processes inspire groundbreaking innovations in medical device technology. From jellyfish to fireflies, the natural world has provided invaluable tools that are transforming diagnostics and research.
-

In this episode of Bio Break, Joris van der Heijden and Nick Allan discuss an advanced category of combination devices: drug activation devices. Unlike drug delivery devices that transport medication to specific locations, drug activation devices ensure the drug becomes active precisely where it is needed in the body, reducing systemic side effects and enhancing therapeutic effectiveness.
-

In this engaging episode of Bio Break, Nick and Joris dive into the complexities of clinical trials as a critical component of medical product development. Whether you're a developer embarking on your first trial or a seasoned professional seeking guidance, this discussion provides actionable insights and resources to streamline the process.
-
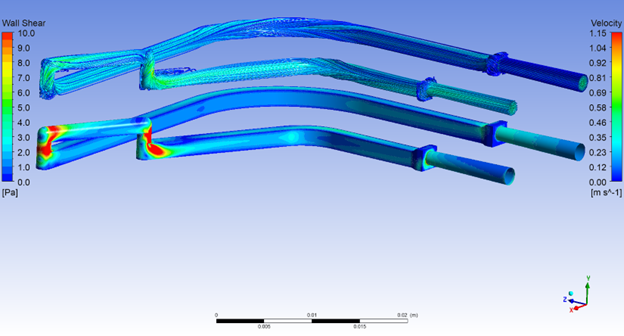
Drug Delivery Solutions for Cell and Gene Therapy (CGT) Challenges explores effective delivery devices and solutions for advanced CGT therapies and their unique challenges.
-
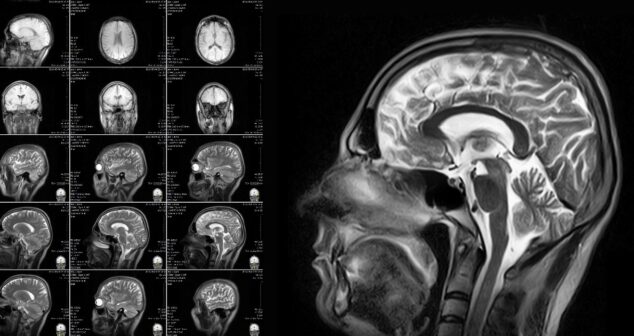
Drug Delivery System Imaging Technology options include MRI, CT, and Ultrasound. This article highlights advantages and disadvantages for each technology in guiding drug delivery.