Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
Medical device CFD simulations allow you to generate images and videos with little effort to predict and optimize fluid and thermal paths.
-

Garbage In, Garbage out…
-
The importance of medical device performance specifications and effectiveness goals are often overlooked in the design process.
-

The tricorder is the ultimate objective of diagnostic medical device development. But is it Medical Device fact or Fiction?
-

StarFish Medical device development videos viewing is up 38% in 2014. Countdown of the Top 10 most viewed videos in 2014.
-

Most read StarFish Medical Device Development Blogs of 2014: regulatory information, analysis, testing, design, career path, and finances.
-
Staffing a Medical Device Start-Up: shortlist of job descriptions required to develop a complex, novel medical device.
-
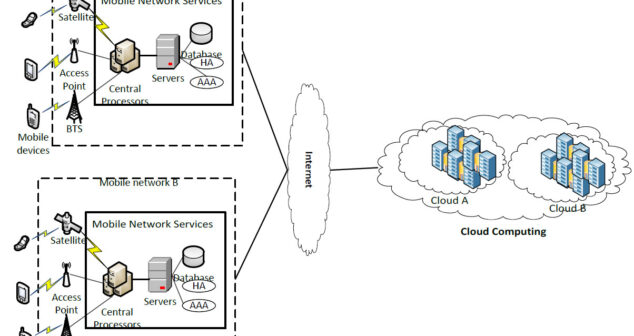
Privacy and cloud-based mobile apps integrated into mobile medical apps. Summary of privacy considerations.
-

Prototyping electronic systems: Tips for avoiding rework include using developmental test boards while creating early prototypes for projects.