Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

We explore the world of brain-computer interfaces (BCIs) and the challenges of capturing thought into action. Mark Drlik and Ariana Wilson walk through how these systems translate brain activity into control signals for devices—without needing surgical implants.
-
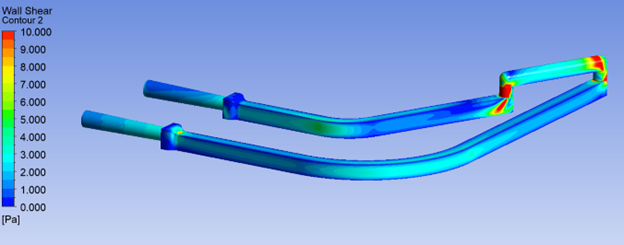
The impact of shear stress is critical to effectively design medical devices that handle biological fluids such as proteins or cell culture media. For example, non-physiological shear stress (NPSS) on blood is a key factor because hemolysis (cell rupture) could occur due to accumulated stress.
-

The costs of early-stage medical device development in North America and Europe continue to rise. Increasing technical complexity and the compounding costs of nonclinical and clinical evaluations are driving this trend.
-

Today, 85% of the top 50 healthcare companies use Computational Modeling and Simulation (CM&S) to develop their products and processes. Whether it’s refining overall device parameters or optimizing critical requirements, engineering simulations help reduce development timelines and enhance design exploration.
-

Paul Charlebois and Eric Olson explore how contextual inquiry in MedTech drives smarter product design. By observing how users interact with devices in real settings, product teams can gather early insights that shape usability, adoption, and safety—long before development begins.
-
Computational Modeling and Simulation (CM&S) for medical devices has become a pivotal tool across the medical device industry, complementing and often enhancing traditional bench testing and clinical studies.
-
Ariana Wilson and Mark Drlik dive into how the FDA is adopting artificial intelligence to modernize its regulatory processes. With a new chief AI officer in place and rumors of collaboration with OpenAI, the agency is taking major steps to automate review workflows and improve efficiency.
-

A theranostic wound dressing does more than cover a cut—it actively detects infection and delivers targeted treatment.
-

In this episode of MedDevice by Design, we follow the development journey of a transformative chest therapy device for cystic fibrosis patients. Host Mark Drlik introduces a voice coil prototype from an early-stage project that would eventually evolve into the Hill-Rom Monarch—a commercial system delivering high-frequency chest wall oscillation therapy.