Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Top 10 2015 StarFish Medical videos help connect viewers with employees they may only know from reading or phone calls.
-

Top 2015 medical device blogs written by StarFish Medical expert employees sharing their knowledge, tips, and tools.
-

3 digital health barriers that explain why Digital Health has been around since the 80s, but progress is not so inspiring.
-

Benefits reaped from working with design and development firms that co-locate medical device manufacturing.
-

Working with a contract manufacturer questions to help choose the right partner and get the most out of a contract manufacturing relationship.
-

3 trade-offs to consider when launching first builds of low volume medical devices.
-

Contract manufacturer minimum volume framework and benefits to consider to reduce challenges for new medical device companies.
-
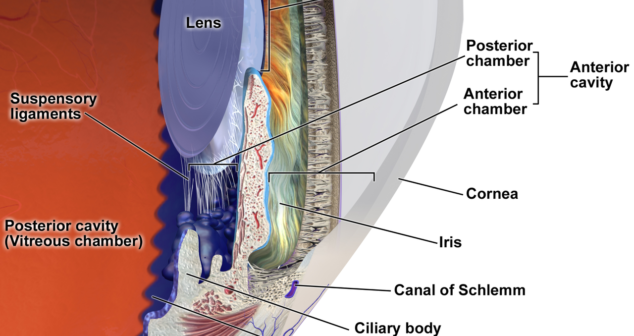
Understanding eye accommodation and surrounding tissue materials properties for accommodative intraocular lenses (AIOLs).
-

Expedited Access Pathway (EAP) is promising for timely approval and access to devices for life-threatening or irreversibly debilitating conditions.