Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Reduce costs by reusing IVD Verification Test equipment for verification of optical components as part of manufacturing quality control.
-

Many (IVDs) employ optical techniques to sense. How to develop low cost IVDs suitable for Point of Care use – optical sensing.
-

Medical Device Design Consulting similarities with boatyard operations pondered while prepping sailboat for annual Pacific island sail.
-

PCB Design Best Practices for Medical Devices shares tips and advice for designing Printed Circuit Boards.
-
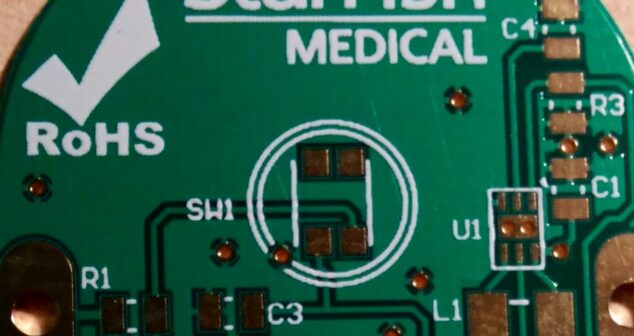
PCB Design Best Practices that increase the chances of success developing a medical device from Principal designer of printed circuit boards.
-

Engineering expertise in design and development lessons learned in the years since school – both in and out of the medical device industry.
-

Benefits of best sourcing medical device team to maximize internal resources and supplement core competencies.
-

Results of StarFish client satisfaction survey with a large group that included customers and prospects using one word descriptions.
-

Regulatory affairs expert explains how to write a best practice ISO 9000 Medical Device Quality Manual using 3 simple rules.