
6 Drug Delivery Routes in the Eye
The human eye is an extremely delicate organ, often prone to irritation, dryness and various diseases, such as glaucoma, cataracts, keratoconus, age-related macular degeneration, and many others. These ocular clinical conditions also affect patients’ quality of life. According to the World Health Organization, every five seconds a person in the world becomes blind; in addition, about 1.3 billion people suffer from vision impairments. This blog describes six approaches to improve drug delivery routes in the eye for more targeted therapy and improved outcomes.
Nowadays, eye drops are the most widely used ocular drug delivery system; indeed, it is estimated that about 90% of ophthalmic drugs are administered in the form of eye drops. Although this route of administration is well-accepted by patients, the bioavailability of drugs administered with topical eye drops is very low. Numerous anatomical constraints, such as the corneal epithelium, blood–aqueous and blood–retinal barriers, hinder the ocular permeation of the drug. There are several approaches to improve ocular drug delivery, depending on the target area (e.g., anterior or posterior segment of the eye). The figure below figure shows sites where drugs can be introduced for more targeted therapy and improved outcomes.
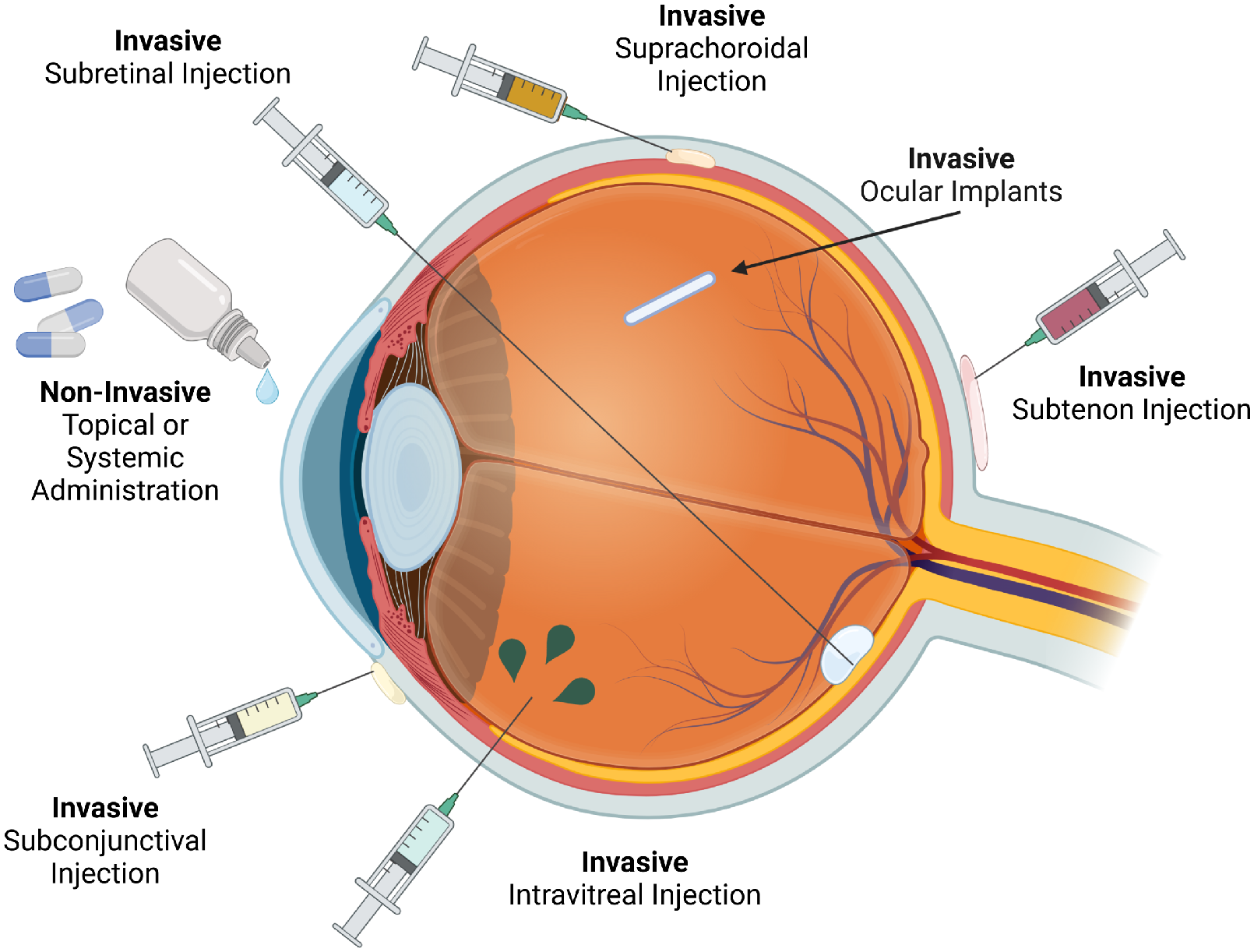
Topical Drug Delivery
Topical drug delivery (e.g., eye drops, gels, ointments, contact lenses) is the most common method for treating anterior segment eye diseases such as glaucoma, dry eye, and infections. This method is preferred due to its ease of use, patient compliance, and non-invasiveness. There are multiple challenges associated with this type of drug delivery which include but are not limited to low bioavailability, rapid drug clearance, limited drug penetration, patient compliance and preservative toxicity.
Many strategies are under development to overcome these challenges that include hydrogel and nanocarrier based drugs, more bioavailable formulations and drug loaded contact lenses. This last approach seems promising due to patient acceptability (consider that a large segment of society currently uses contact lens-based products). As of March 2025, ACUVUE® Theravision™ with Ketotifen by Johnson & Johnson Vision Care is the first and only drug-eluting contact lens approved by the U.S. Food and Drug Administration (FDA). This daily disposable lens is designed to prevent ocular itch due to allergic conjunctivitis while providing vision correction for individuals.
Intravitreal Injection
Intravitreal injection is the direct delivery of a drug into the vitreous humor (the gel-like substance inside the eye). This method is used to treat retinal diseases and conditions affecting the posterior segment of the eye, such as macular degeneration, diabetic retinopathy, and retinal vein occlusion. The advantages of this delivery route that include: high drug concentration at the target site, avoidance of blood-retinal barrier issues, improved bioavailability compared to systemic administration and minimized systemic side effects – localized treatment reduces systemic drug exposure.
Common uses include anti-VEGF agents, anti-inflammatories, antibiotics & antifungals (for endophthalmitis). Usually, 30G to 32G needles are used to minimize trauma and discomfort.
Currently, products that are approved for ocular use and administered through intravitreal injection include SUSVIMO® (ranibizumab injection) and EYLEA® (aflibercept).
Subconjunctival Injection
Subconjunctival injection is the administration of a drug into the space between the conjunctiva and the sclera (the white part of the eye). This method allows for localized drug delivery, enhancing absorption and prolonging drug action compared to topical eye drops. Advantages include higher drug bioavailability, prolonged drug retention, less systemic absorption and targeted delivery. However, there are a few challenges and limitations to this method which include: risk of infection, pain and discomfort and conjunctival scarring. This method is most used for treatments and therapies listed below:
- Anti-VEGF Therapy (Experimental Uses) – Investigated for delivering bevacizumab or ranibizumab to treat conditions like diabetic retinopathy.
- Antibiotics – Used in severe bacterial infections (e.g., cephalosporins, gentamicin for corneal ulcers).
- Anti-inflammatory Therapy – Steroids like triamcinolone or dexamethasone for uveitis, scleritis, or post-surgical inflammation.
- Antifungal Medications – Amphotericin B or voriconazole for fungal keratitis.
- Glaucoma Treatment – Drug-loaded implants (e.g., sustained-release bimatoprost) to lower intraocular pressure.
While subconjunctival injections are utilized in clinical practice, especially for delivering anti-inflammatory agents, there are currently no FDA-approved medications specifically labeled for this route of administration. Healthcare providers may use certain drugs off-label via subconjunctival injection based on clinical judgment and individual patient needs.
Suprachoroidal Injection
Suprachoroidal injection delivers drugs into the suprachoroidal space (SCS), which lies between the sclera and the choroid. This targeted approach enhances drug penetration into the retina and choroid while minimizing exposure to non-ocular tissues. Advantages of Suprachoroidal Injection are same as intravitreal injection with the added benefit of reduced risk of vitreous complications because it avoids direct injection into the vitreous, lowering risks like endophthalmitis or retinal detachment. Some challenges include limited approved drugs and the learning curve for clinicians.
Clearside Biomedical in collaboration with Bausch + Lomb has developed an injection therapy that is FDA approved and commonly known as XIPERE® (Triamcinolone Acetonide Injectable Suspension), a corticosteroid that reduces inflammation when delivered into the suprachoroidal space. Other therapies in clinical trials, such as CLS-AX and some AAV-based gene therapies, may prove promising for anti-VEGF treatments and other retinal disorders.
The SCS Microinjector® (Clearside Biomedical) is a specialized injection device designed for precise suprachoroidal space delivery using custom 900-micron needle to ensure accurate delivery into the suprachoroidal space and a minimally invasive design that reduces injection-related trauma.
Intracameral Injection
Intracameral injection is the administration of a drug directly into the anterior chamber of the eye, which is the fluid-filled space between the cornea and the iris. This method provides targeted drug delivery to the anterior segment, allowing for rapid and localized therapeutic effects.
Common uses of intracameral injection include endophthalmitis prevention in cataract surgery, glaucoma management, intraoperative mydriasis (pupil dilation) and inflammation control, and steroid delivery for post-surgical inflammation. 27G to 30G needles that are about 13mm in length can be used for these kinds of injections. Potential complications involved with this method include corneal toxicity and sudden pressure increase.
Intracameral injections offer a safe and effective way to deliver antibiotics, anti-inflammatory agents, and glaucoma treatments directly into the anterior chamber. Omidria® (phenylephrine/ketorolac) is a notable FDA-approved product that has improved glaucoma management and cataract surgery outcomes.
Ocular Implants
Ocular implants are biodegradable or non-biodegradable drug delivery devices designed to release medication directly into the eye over an extended period. They help treat chronic eye diseases while reducing the need for frequent injections or topical eye drops. With continued advancements, implants could replace frequent injections and eye drops, significantly improving patient outcomes. Challenges associated with ocular implants include surgical insertion is required, risk of infection & complications can lead to possible endophthalmitis, IOP (intra-ocular pressure) spikes, and high cost compared to traditional drug delivery. Potential approaches for future treatments include gene therapy-based implants, smart drug-release systems, and bioengineered polymers – biodegradable materials for extended-release with fewer complications.
Other fascinating products on market include Ozurdex® – Dexamethasone biodegradable implant, Iluvien® – Fluocinolone acetonide non-biodegradable implant, and Retisert® – Fluocinolone implant for chronic uveitis. They are a few of many treatments that deserve special mention that are designed for intravitreal space.
In summary, there are pros and cons of each type of delivery method. Due to anatomical and physiological barriers, such as tear turnover, blinking, corneal epithelium, and blood-ocular barriers, drug delivery into eye is challenging. Ultimately this review demonstrates that there are a large range of drug delivery routes in the eye options available for the treatment of many conditions. Selecting the appropriate delivery route is driven by the anatomical features of the treatment site and the mechanism of action of the therapeutic delivered.
Disha Patel is a Research Scientist at StarFish Medical.
Learn how patient imaging data can be a powerful and robust source for developing anatomically accurate and functional 3D models.
Related Resources


Nick and Nigel walk through how sterile disposables are processed and verified before they reach the field.

The FDA agentic AI is making headlines after the agency announced its own internal AI review tool. In this episode of MedDevice by Design, Ariana and Mark discuss what this could mean for medical device submissions and regulatory efficiency.

The sandwich ELISA assay is one of the most common ELISA formats used in diagnostics. Nick and Nigel walk through the method step by step using simple visuals and plain language.