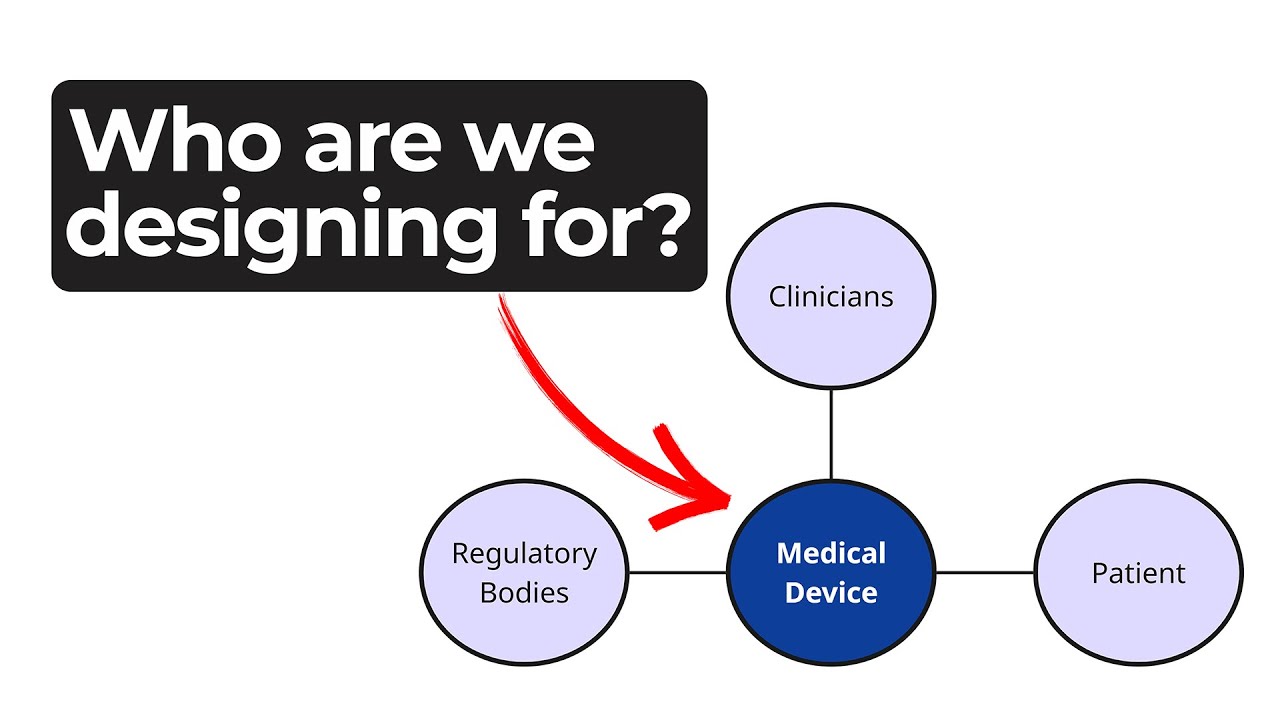
Understanding Medical Device Stakeholders
When designing a new product, identifying medical device stakeholders is a vital first step. As Ariana Wilson and Mark Drlik explain in this episode of MedDevice by Design, every phase of a device’s life cycle involves different people with distinct needs—from clinicians and patients to service technicians and regulatory bodies. Understanding who these stakeholders are and what they value helps create more effective and sustainable designs.
Mapping user needs across the product life cycle
Stakeholders influence a product’s success through purchase, adoption, use, advocacy, and sometimes even rejection. By mapping all involved parties—assembly teams, procurement, distributors, maintenance staff, and end users—designers can visualize where interests overlap or diverge. This mapping process allows teams to identify conflicting needs early and guide design tradeoffs before they become barriers to usability or compliance.
Balancing conflicting stakeholder needs
As Wilson points out, user needs often conflict. A facilities manager may prioritize infection control, while a biomed technician may need quick access for repairs. These needs seem opposed, but they stem from shared goals: safety and efficiency. Recognizing the core requirement behind each helps designers uncover creative ways to satisfy both. Drlik adds that thoughtful design, combined with iterative feedback, can resolve these tensions and sometimes inspire entirely new solutions.
From user insights to design innovation
By interpreting and negotiating stakeholder needs, designers transform requests into actionable design requirements. This process keeps teams focused on solving real problems rather than simply responding to feature requests. As the discussion highlights, creative solutions often emerge when designers fully understand the perspectives of all medical device stakeholders.
Enjoying MedDevice by Design? Sign up to get new episodes sent to your inbox.
Related Resources

The FDA agentic AI is making headlines after the agency announced its own internal AI review tool. In this episode of MedDevice by Design, Ariana and Mark discuss what this could mean for medical device submissions and regulatory efficiency.
In this episode of MedDevice by Design, Ariana Wilson and Mark Drlik explore what sits beneath that progress, focusing on how these therapies are delivered and why delivery remains one of the hardest problems to solve.

Ariana Wilson and Mark Drlik break down medical vs wellness devices and explain why two products with identical hardware can fall into completely different regulatory categories.

Ariana Wilson sits down with Mark Drlik to unpack why reprocessing is often one of the hardest challenges engineers face during development.