Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Nick and Joris dive into the fascinating world of freeze-drying, exploring how this process extends shelf life and maintains the integrity of various products—including reagents used in in vitro diagnostics and even instant coffee!
-

In this episode of Bio Break, Nick and Joris discuss the fascinating world of real-time imaging for targeted drug delivery. When delivering drugs to precise locations in the body, how do we ensure they reach the right spot? The answer lies in medical imaging technologies such as MRI, CT, and ultrasound, which play a crucial role in guiding complex drug delivery devices.
-

ASTM D4169 Options for this standard test method of performance testing shipping containers and packaging systems are explored.
-

What are the most important medical device success factors during development and manufacturing? StarFish employees from QA/RA, NPI, Optics, Computational Analysis, Project Management and Manufacturing answer that question with the factors they deem most important for their area of expertise.
-
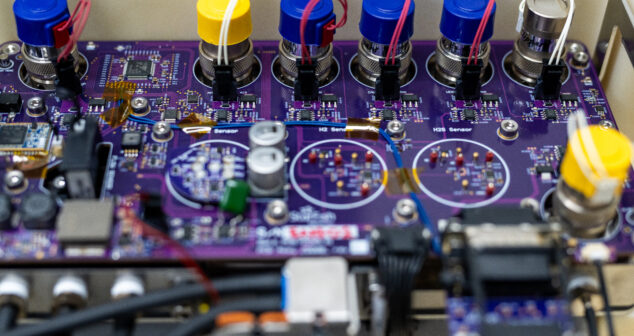
Getting a PCB (Printed Circuit Board) for a medical device right the first time is almost impossible. Datasheets can be misleading, or assumptions and architectures change. As a result, modifications are almost inevitable. Sometimes the modification is as simple as swapping resistors or adding capacitors. Other times it involves tacking on new circuits you had no idea you needed.
-

This blog explores key highlights of the Chemical Analysis for Biocompatibility Assessment of Medical Devices draft guidance, focusing on its scope, testing methodologies, and recommendations for reporting. In September 2024, the U.S. Food and Drug Administration (FDA) issued a draft guidance titled Chemical Analysis for Biocompatibility Assessment of Medical Devices.
-

Nick and Joris explore the fascinating world of repurposing existing medical device technologies for new market sectors. As engineers and innovators, we often focus on creating brand-new solutions, but what about leveraging tried-and-true technologies to expand into untapped markets? This strategy not only opens doors to new revenue streams but also maximizes the potential of existing innovations.
-

In the webinar "Grand Challenges in Neuroscience" from January 21, 2025, Dr. Jacob Hooker, Lurie Family Professor of Radiology and Scientific Director at the Lurie Center for Autism at Massachusetts General Hospital, joins Nick Allan, Bio Services Manager at StarFish Medical, to discuss some of the biggest hurdles in neuroscience today. The conversation explores the complex interplay between chemistry, biology, and medical device engineering, offering insights into the latest innovations in neurotherapeutics, molecular imaging, and non-invasive drug delivery technologies.
-

Advancements in neuroscience are reshaping our understanding of the human brain, but significant challenges remain in translating scientific breakthroughs into effective treatments….