Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Ariana and Mark walk through FDA-approved options and explain how to select the right one for your product. From metals to plastics and electronics, not all devices can handle the same process.
-
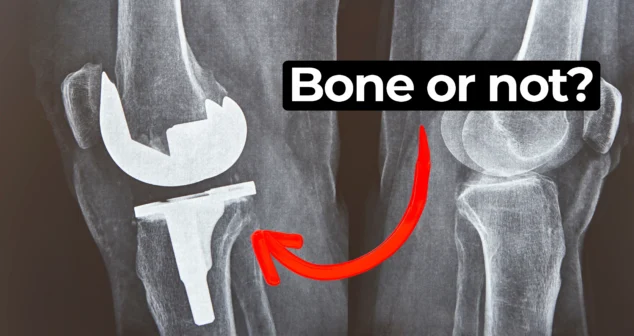
In this episode of MedDevice by Design, Ariana and Mark dive into the biomechanics and materials science behind osseointegration for implants.
-

Ariana and Mark explore how accommodative intraocular lens technology may one day restore natural vision for people who require cataract surgery or suffer from presbyopia. As Mark shares, traditional bifocals are not ideal, and new lens solutions may offer better outcomes.
-

We explore the world of brain-computer interfaces (BCIs) and the challenges of capturing thought into action. Mark Drlik and Ariana Wilson walk through how these systems translate brain activity into control signals for devices—without needing surgical implants.
-
Mark and Ariana explore the surprising versatility of barium sulfate—a material used widely in both diagnostic procedures and medical device manufacturing. While many recognize it as the contrast agent you drink before an X-ray, it’s also a key additive that enhances plastic components across the healthcare industry.
-
We explore a groundbreaking shift in how Alzheimer’s disease may soon be diagnosed. Instead of relying on invasive spinal taps or costly PET scans, researchers have developed a blood test that detects key proteins associated with the disease—offering a more accessible and patient-friendly screening method.
-

We explore how breath testing in medical devices is transforming diagnostics. Mark Drlik walks through how this technology supports everything from roadside impairment detection to gastrointestinal analysis.
-

Mark Drlik and Ariana Wilson introduce the fascinating world of ingestible capsules—tiny, swallowable medical devices that are revolutionizing gastrointestinal health monitoring and targeted therapy.
-
Ariana Wilson and Mark Drlik dive into how the FDA is adopting artificial intelligence to modernize its regulatory processes. With a new chief AI officer in place and rumors of collaboration with OpenAI, the agency is taking major steps to automate review workflows and improve efficiency.