Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Health Canada improvements: Getting new devices to market, strengthen monitoring and follow-up and provide more information to Canadians.
-

Creative Human Factors Processes for COVID-19 solutions to combat logistical hurdles for human factors research and evaluation activities.
-

FDA provides a roadmap to follow for premarket submission in their guidance on Content and Format of Non-Clinical Bench Performance Testing.
-
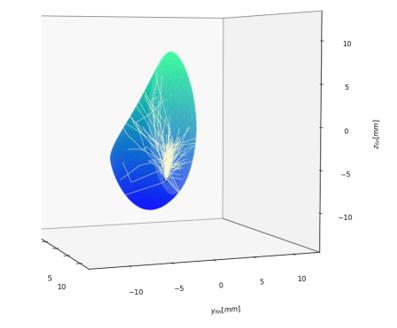
Monte Carlo Simulation for Biomedical Device Design: A common approach to simulate scattering is the Monte Carlo technique.
-
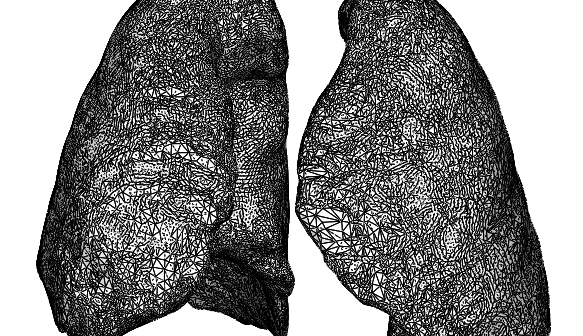
Create better functional models from anatomical scans with free tool for easy manipulation, simplification and cleaning up of mesh files.
-

Clean or Sterile? If a medical device does not need to be sterile, how clean does it need to be? In-depth review of terms and requirements.
-

Biological sciences medical device breakthroughs for this decade have a solid base to build upon. Here are our top five picks for the 2020's.
-

Translating point of care assays into a simple, low cost and easy to use microfluidic cartridge is straightforward with these six steps.
-

Dr. Katherine Elvira speaks about her work developing lab-on-a-chip (microfluidic) technologies to investigate how drugs enter human cells.